Ronan Kelly, MD, MBA,
The Charles A. Sammons Cancer Center,
Baylor University Medical Center, Dallas, Texas*
Content sponsored by: Bristol Myers Squibb
*Dr Kelly was compensated by BMS for his contribution in drafting this article.
Introduction: Overview of gastroesophageal adenocarcinoma
Gastroesophageal adenocarcinomas consist of a heterogeneous group of tumors, including gastric cancer (GC), gastroesophageal junction cancer (GEJC), and esophageal adenocarcinoma (EAC), all of which are aggressive malignancies with poor outcomes.3-6 The aggressive natures of GC and EAC may contribute to their respective statuses as two of the most common causes of cancer-related death globally.7
 Checkmate 649 led to the approval of nivolumab (OPDIVO) + chemotherapy as the first chemoimmunotherapy combination for all eligible patients with HER2-negative† GC/GEJC/EAC regardless of PD-L1 status.1,8,9 Prior to this approval, chemotherapy was the only available 1L treatment option for metastatic GC/GEJC/EAC.10 Furthermore, to date, Checkmate 649 has the longest follow-up survival data in GC vs chemotherapy for any I-O–based regimen with a minimum follow-up of 48.1 months (median of 59.3 months), and showed durable survival data with OPDIVO + chemotherapy in GC/GEJC/EAC.1,2,11 OPDIVO can be given q2w or q3w, which synchronizes with the q2w FOLFOX and q3w CapeOx dosing schedules.1 “The flexible dosing schedule of OPDIVO has made it more convenient to integrate into my clinical practice,” stated Dr. Kelly.
Checkmate 649 led to the approval of nivolumab (OPDIVO) + chemotherapy as the first chemoimmunotherapy combination for all eligible patients with HER2-negative† GC/GEJC/EAC regardless of PD-L1 status.1,8,9 Prior to this approval, chemotherapy was the only available 1L treatment option for metastatic GC/GEJC/EAC.10 Furthermore, to date, Checkmate 649 has the longest follow-up survival data in GC vs chemotherapy for any I-O–based regimen with a minimum follow-up of 48.1 months (median of 59.3 months), and showed durable survival data with OPDIVO + chemotherapy in GC/GEJC/EAC.1,2,11 OPDIVO can be given q2w or q3w, which synchronizes with the q2w FOLFOX and q3w CapeOx dosing schedules.1 “The flexible dosing schedule of OPDIVO has made it more convenient to integrate into my clinical practice,” stated Dr. Kelly.
†Indication has no restriction on HER2 status; trial included HER2-negative patients and patients with unknown HER2 status, while excluding those with known HER2-positive status.1
OPDIVO + chemotherapy in 1L metastatic GC/GEJC/EAC
With the longest follow-up survival data in GC vs chemotherapy for any I-O–based regimen and durable survival data in GC/GEJC/EAC, OPDIVO + fluoropyrimidine- and platinum-containing chemotherapy is currently FDA-approved in 1L metastatic non–HER2-positive† GC/GEJC/EAC, regardless of PD-L1 status (no testing required).1,2,9 The approval of this combination was based on the results of Checkmate 649, a global phase 3 study in patients with 1L metastatic GC/GEJC/EAC.1,8 Key exclusion criteria included known HER2-positive status and untreated CNS metastases.8 The study recruited all eligible patients regardless of PD-L1 expression.1,8
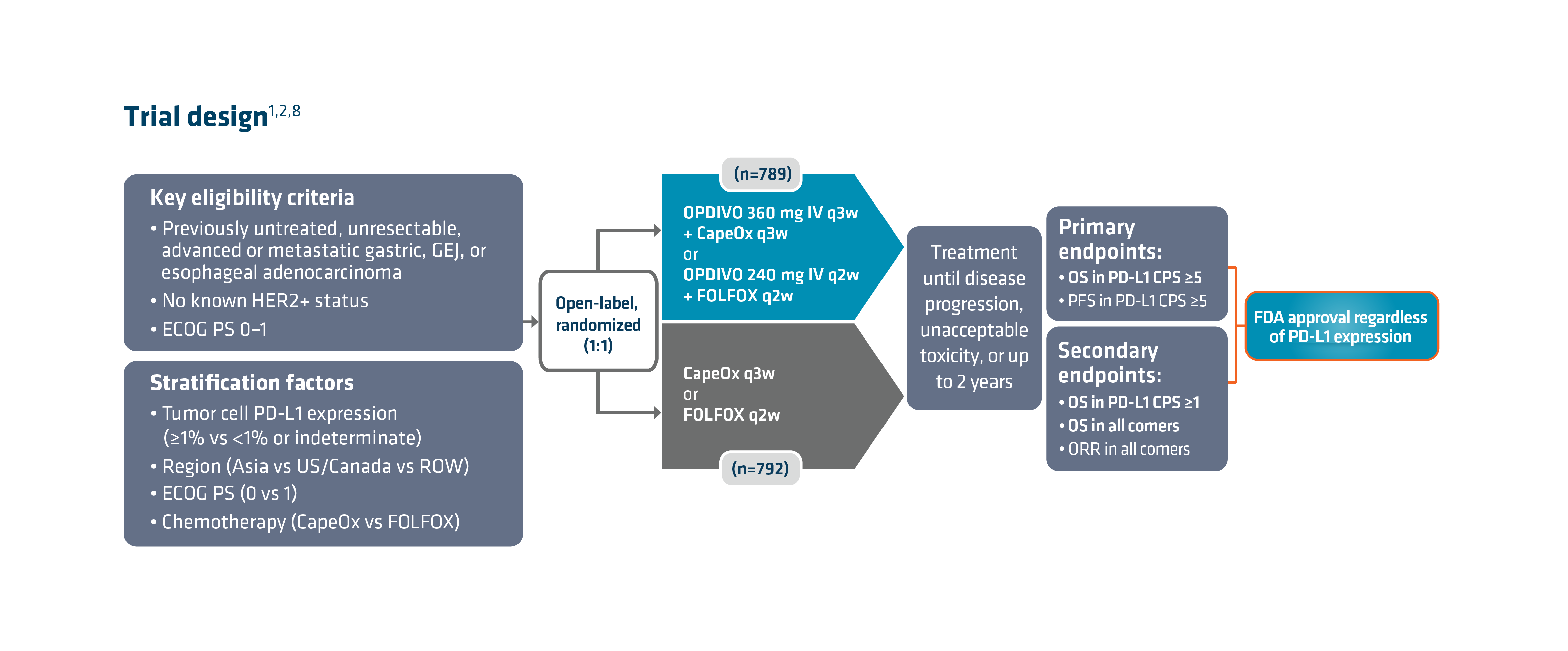 Checkmate 649 enrolled 1581 patients randomized 1:1 to receive either OPDIVO + chemotherapy (n=789) or chemotherapy alone (n=792). The dual primary endpoints were OS and PFS in PD-L1 CPS ≥5. OS in PD-L1 CPS ≥1 and in all-comers were secondary endpoints, but were powered to measure statistical significance through hierarchical analysis. Baseline characteristics were consistent among all randomized patients and patients with PD-L1 CPS ≥5. Checkmate 649 was the first phase 3 trial to achieve positive results in the evaluation of a PD-1 inhibitor in combination with FOLFOX or CapeOx, allowing for synchronized I-O dosing options with the preferred chemotherapy.8
Checkmate 649 enrolled 1581 patients randomized 1:1 to receive either OPDIVO + chemotherapy (n=789) or chemotherapy alone (n=792). The dual primary endpoints were OS and PFS in PD-L1 CPS ≥5. OS in PD-L1 CPS ≥1 and in all-comers were secondary endpoints, but were powered to measure statistical significance through hierarchical analysis. Baseline characteristics were consistent among all randomized patients and patients with PD-L1 CPS ≥5. Checkmate 649 was the first phase 3 trial to achieve positive results in the evaluation of a PD-1 inhibitor in combination with FOLFOX or CapeOx, allowing for synchronized I-O dosing options with the preferred chemotherapy.8
There are warnings and precautions associated with OPDIVO to keep in mind. These include severe and fatal immune-mediated adverse reactions, infusion-related reactions, complications of allogeneic hematopoietic stem cell transplantation, embryo-fetal toxicity, and increased mortality in patients with multiple myeloma when OPDIVO is added to a thalidomide analogue and dexamethasone, which is not recommended outside of controlled clinical trials.1 Additional information related to warnings and precautions can be found here .
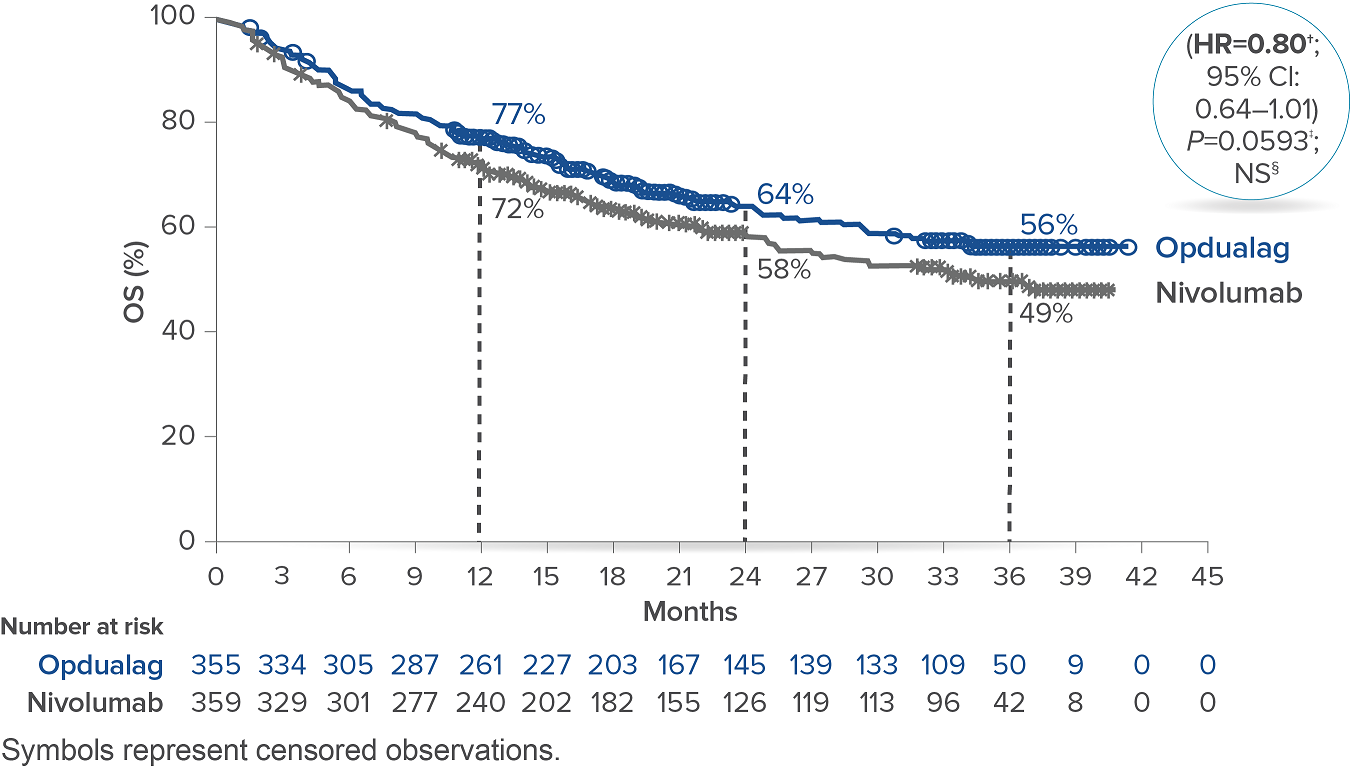
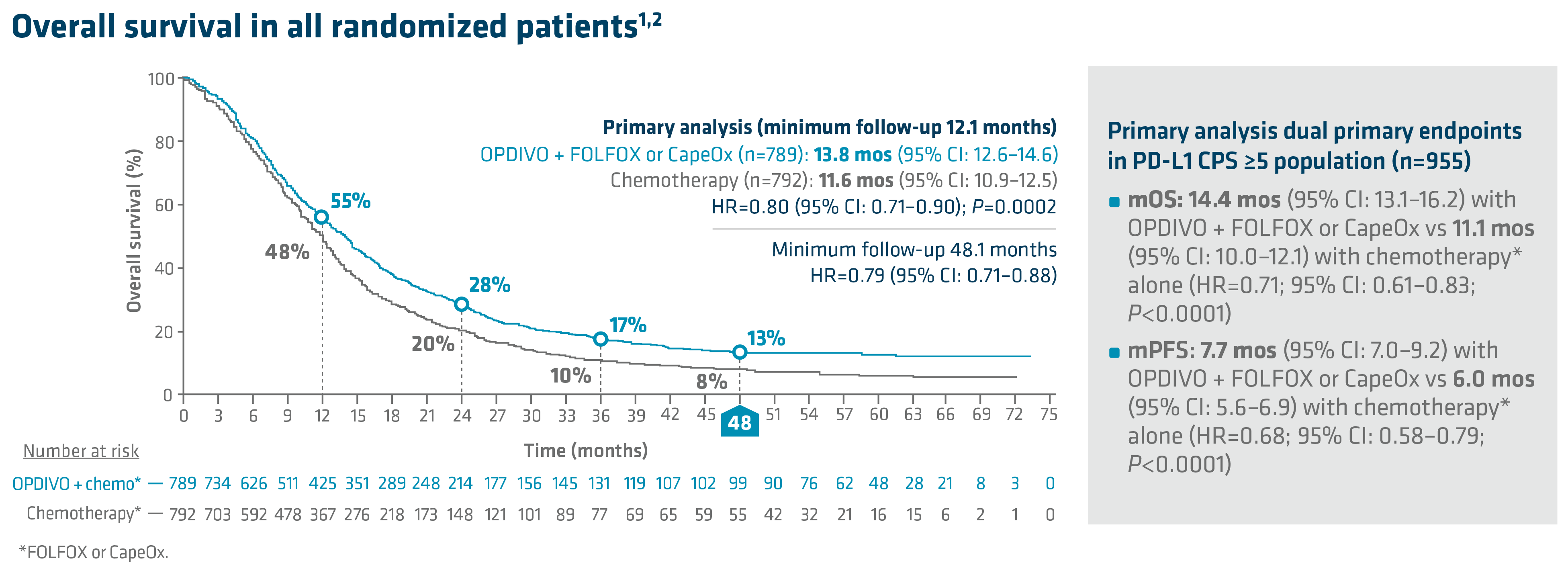 In the primary analysis (minimum follow-up of 12.1 months), OPDIVO + chemotherapy demonstrated superior OS in all randomized patients and patients with PD-L1 CPS ≥5, as compared to chemotherapy alone. In all randomized patients, mOS was 13.8 mos with OPDIVO + chemotherapy vs 11.6 mos with chemotherapy (HR=0.80; 95% CI: 0.71–0.90; P=0.0002). In patients with PD-L1 CPS ≥5 (n=955), mOS was 14.4 mos with OPDIVO + chemotherapy vs 11.1 mos with chemotherapy (HR=0.71; 95% CI: 0.61–0.83; P<0.0001).1 The 12-month OS rate in all randomized patients was 55% with OPDIVO + chemotherapy vs 48% with chemotherapy.8 “In my opinion, clinical trial data with OPDIVO + chemotherapy was a landmark. For the first time in a non–HER2-positive population, patients were able to break through the 1-year mOS barrier,” explained Dr. Kelly.
In the primary analysis (minimum follow-up of 12.1 months), OPDIVO + chemotherapy demonstrated superior OS in all randomized patients and patients with PD-L1 CPS ≥5, as compared to chemotherapy alone. In all randomized patients, mOS was 13.8 mos with OPDIVO + chemotherapy vs 11.6 mos with chemotherapy (HR=0.80; 95% CI: 0.71–0.90; P=0.0002). In patients with PD-L1 CPS ≥5 (n=955), mOS was 14.4 mos with OPDIVO + chemotherapy vs 11.1 mos with chemotherapy (HR=0.71; 95% CI: 0.61–0.83; P<0.0001).1 The 12-month OS rate in all randomized patients was 55% with OPDIVO + chemotherapy vs 48% with chemotherapy.8 “In my opinion, clinical trial data with OPDIVO + chemotherapy was a landmark. For the first time in a non–HER2-positive population, patients were able to break through the 1-year mOS barrier,” explained Dr. Kelly.
Durable survival data was observed for this OPDIVO-based regimen vs chemotherapy alone in GC/GEJC/EAC. The follow-up analysis at 48.1 months reported a mOS of 13.7 mos (95% CI: 12.4–14.5) with OPDIVO + chemotherapy vs 11.6 mos (95% CI: 10.9–12.5) with chemotherapy in all randomized patients (HR=0.79; 95% CI: 0.71–0.88), and 14.4 mos (95% CI: 13.1–16.2) with OPDIVO + chemotherapy vs 11.1 mos (95% CI: 10.1–12.1) with chemotherapy in patients with PD-L1 CPS ≥5 (HR=0.70; 95% CI: 0.61–0.81). The 48-month OS rate was 13% vs 8% for OPDIVO + chemotherapy vs chemotherapy, respectively, in all randomized patients.2
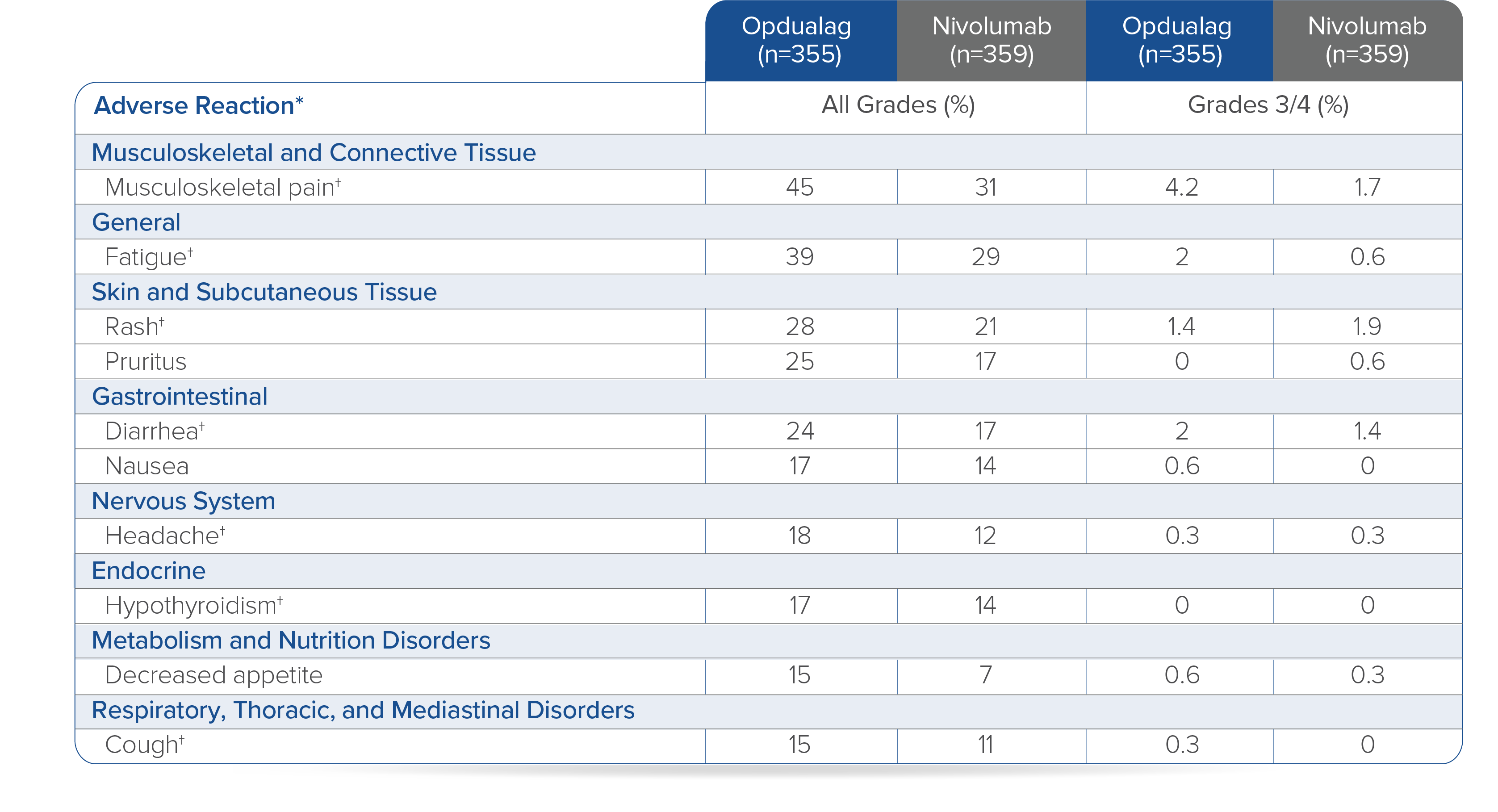
In Checkmate 649, the most common adverse reactions reported in ≥20% of patients treated with OPDIVO in combination with chemotherapy were peripheral neuropathy, nausea, fatigue, diarrhea, vomiting, decreased appetite, abdominal pain, constipation, and musculoskeletal pain. OPDIVO and/or chemotherapy were discontinued in 44% of patients and at least one dose was withheld in 76% of patients due to an adverse reaction. Serious adverse reactions occurred in 52% of patients treated with OPDIVO in combination with chemotherapy. The most frequent serious adverse reactions reported in ≥2% of patients treated with OPDIVO in combination with chemotherapy were vomiting (3.7%), pneumonia (3.6%), anemia (3.6%), pyrexia (2.8%), diarrhea (2.7%), febrile neutropenia (2.6%), and pneumonitis (2.4%). Fatal adverse reactions occurred in 16 (2.0%) patients who were treated with OPDIVO in combination with chemotherapy; these included pneumonitis (4 patients), febrile neutropenia (2 patients), stroke (2 patients), gastrointestinal toxicity, intestinal mucositis, septic shock, pneumonia, infection, gastrointestinal bleeding, mesenteric vessel thrombosis, and disseminated intravascular coagulation.1
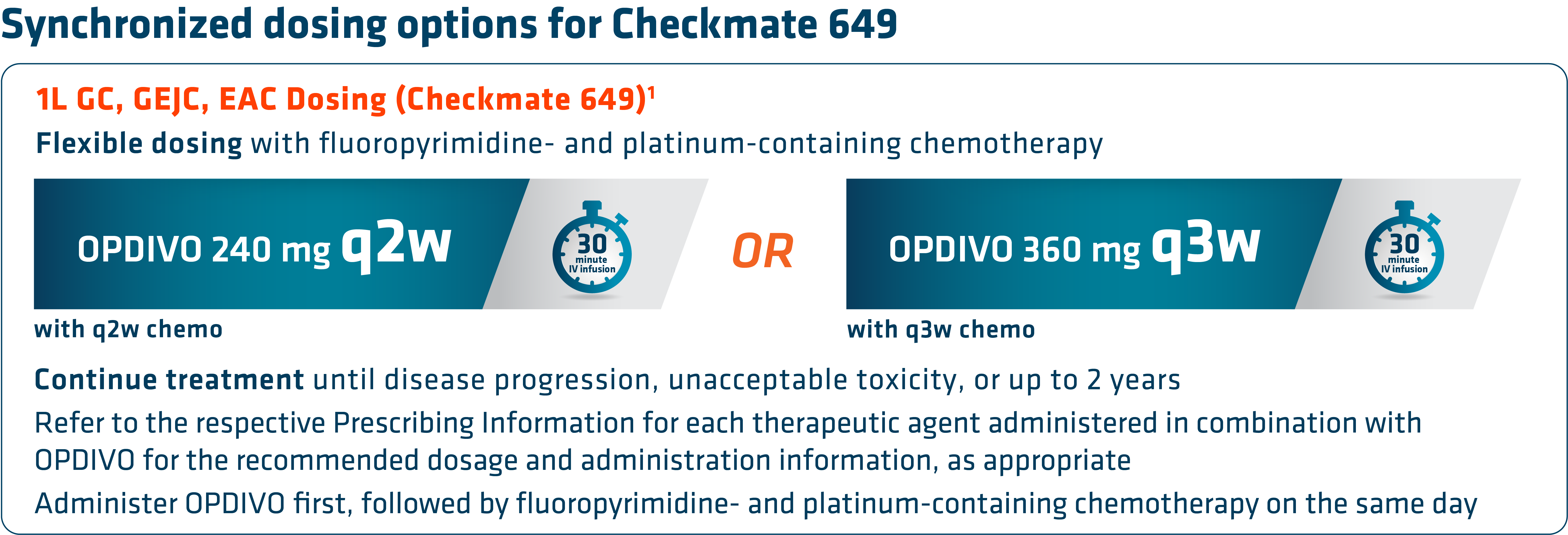
An additional characteristic of OPDIVO is its flexible dosing schedule. Based on both the FDA-approved label and Checkmate 649 trial design, OPDIVO offers flexible synchronized dosing options based on chemotherapy preference, and “in my experience, allows scheduling according to the patient and clinician preference,” stated Dr. Kelly. Checkmate 649 evaluated OPDIVO (q2w or q3w) in combination with physician’s choice of either FOLFOX given q2w or CapeOx given q3w in the first-line treatment of certain metastatic gastroesophageal cancers. Treatment can be continued until disease progression, unacceptable toxicity, or up to 2 years.1
Summary and conclusions
With the longest follow-up survival data in GC vs chemotherapy for any I-O–based regimen and durable survival data in GC/GEJC/EAC, OPDIVO in combination with fluoropyrimidine- and platinum-containing chemotherapy is an approved 1L treatment option for all eligible patients with non–HER2-positive† GC/GEJC/EAC, regardless of PD-L1 status.1,2 OPDIVO also offers synchronized dosing options to align with preferred chemotherapies, including both FOLFOX and CapeOx, which can be used every 2 or 3 weeks, respectively.1 “I believe Checkmate 649 may act as a new benchmark moving forward and novel therapeutics may be compared against it,” stated Dr. Kelly.
1L=first line; CapeOx=capecitabine and oxaliplatin; CI=confidence interval; CNS=central nervous system; CPS=combined positive score; FOLFOX=leucovorin, fluorouracil, and oxaliplatin; GEJ=gastroesophageal junction; HER2=human epidermal growth factor receptor 2; HR=hazard ratio; I-O=immuno-oncology; IV=intravenous; mo=month; mOS=median OS; mPFS=median PFS; ORR=overall response rate; OS=overall survival; PD-1=programmed death receptor-1; PD-L1=programmed death ligand 1; PFS=progression-free survival; q2w=every 2 weeks; q3w=every 3 weeks; ROW=rest of world.
INDICATION
OPDIVO® (nivolumab), in combination with fluoropyrimidine- and platinum-containing chemotherapy, is indicated for the treatment of adult patients with advanced or metastatic gastric cancer, gastroesophageal junction cancer, and esophageal adenocarcinoma.
OPDIVO (10 mg/mL) is an injection for intravenous use.
IMPORTANT SAFETY INFORMATION
Severe and Fatal Immune-Mediated Adverse Reactions
• Immune-mediated adverse reactions listed herein may not include all possible severe and fatal immune-mediated adverse reactions.
• Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue. While immune-mediated adverse reactions usually manifest during treatment, they can also occur after discontinuation of OPDIVO. Early identification and management are essential to ensure safe use of OPDIVO. Monitor for signs and symptoms that may be clinical manifestations of underlying immune-mediated adverse reactions. Evaluate clinical chemistries including liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment with OPDIVO. In cases of suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate.
• Withhold or permanently discontinue OPDIVO depending on severity (please see section 2 Dosage and Administration in the accompanying Full Prescribing Information). In general, if OPDIVO interruption or discontinuation is required, administer systemic corticosteroid therapy (1 to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroid therapy. Toxicity management guidelines for adverse reactions that do not necessarily require systemic steroids (e.g., endocrinopathies and dermatologic reactions) are discussed below.
Immune-Mediated Pneumonitis
• OPDIVO can cause immune-mediated pneumonitis. The incidence of pneumonitis is higher in patients who have received prior thoracic radiation. In patients receiving OPDIVO monotherapy, immune-mediated pneumonitis occurred in 3.1% (61/1994) of patients, including Grade 4 (<0.1%), Grade 3 (0.9%), and Grade 2 (2.1%).
Immune-Mediated Colitis
• OPDIVO can cause immune-mediated colitis. A common symptom included in the definition of colitis was diarrhea. Cytomegalovirus (CMV) infection/reactivation has been reported in patients with corticosteroid-refractory immune-mediated colitis. In cases of corticosteroid-refractory colitis, consider repeating infectious workup to exclude alternative etiologies. In patients receiving OPDIVO monotherapy, immune-mediated colitis occurred in 2.9% (58/1994) of patients, including Grade 3 (1.7%) and Grade 2 (1%).
Immune-Mediated Hepatitis and Hepatotoxicity
• OPDIVO can cause immune-mediated hepatitis. In patients receiving OPDIVO monotherapy, immune-mediated hepatitis occurred in 1.8% (35/1994) of patients, including Grade 4 (0.2%), Grade 3 (1.3%), and Grade 2 (0.4%).
Immune-Mediated Endocrinopathies
• OPDIVO can cause primary or secondary adrenal insufficiency, immune-mediated hypophysitis, immune-mediated thyroid disorders, and Type 1 diabetes mellitus, which can present with diabetic ketoacidosis. Withhold OPDIVO depending on severity (please see section 2 Dosage and Administration in the accompanying Full Prescribing Information). For Grade 2 or higher adrenal insufficiency, initiate symptomatic treatment, including hormone replacement as clinically indicated. Hypophysitis can present with acute symptoms associated with mass effect such as headache, photophobia, or visual field defects. Hypophysitis can cause hypopituitarism; initiate hormone replacement as clinically indicated. Thyroiditis can present with or without endocrinopathy. Hypothyroidism can follow hyperthyroidism; initiate hormone replacement or medical management as clinically indicated. Monitor patients for hyperglycemia or other signs and symptoms of diabetes; initiate treatment with insulin as clinically indicated.
• In patients receiving OPDIVO monotherapy, adrenal insufficiency occurred in 1% (20/1994), including Grade 3 (0.4%) and Grade 2 (0.6%).
• In patients receiving OPDIVO monotherapy, hypophysitis occurred in 0.6% (12/1994) of patients, including Grade 3 (0.2%) and Grade 2 (0.3%).
• In patients receiving OPDIVO monotherapy, thyroiditis occurred in 0.6% (12/1994) of patients, including Grade 2 (0.2%).
• In patients receiving OPDIVO monotherapy, hyperthyroidism occurred in 2.7% (54/1994) of patients, including Grade 3 (<0.1%) and Grade 2 (1.2%).
• In patients receiving OPDIVO monotherapy, hypothyroidism occurred in 8% (163/1994) of patients, including Grade 3 (0.2%) and Grade 2 (4.8%).
• In patients receiving OPDIVO monotherapy, diabetes occurred in 0.9% (17/1994) of patients, including Grade 3 (0.4%) and Grade 2 (0.3%), and 2 cases of diabetic ketoacidosis.
Immune-Mediated Nephritis with Renal Dysfunction
• OPDIVO can cause immune-mediated nephritis. In patients receiving OPDIVO monotherapy, immune-mediated nephritis and renal dysfunction occurred in 1.2% (23/1994) of patients, including Grade 4 (<0.1%), Grade 3 (0.5%), and Grade 2 (0.6%).
Immune-Mediated Dermatologic Adverse Reactions
• OPDIVO can cause immune-mediated rash or dermatitis. Exfoliative dermatitis, including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug rash with eosinophilia and systemic symptoms (DRESS) has occurred with PD-1/PD-L1 blocking antibodies. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate nonexfoliative rashes.
• Withhold or permanently discontinue OPDIVO depending on severity (please see section 2 Dosage and Administration in the accompanying Full Prescribing Information).
• In patients receiving OPDIVO monotherapy, immune-mediated rash occurred in 9% (171/1994) of patients, including Grade 3 (1.1%) and Grade 2 (2.2%).
Other Immune-Mediated Adverse Reactions
• The following clinically significant immune-mediated adverse reactions occurred at an incidence of <1% (unless otherwise noted) in patients who received OPDIVO monotherapy or were reported with the use of other PD-1/PD-L1 blocking antibodies. Severe or fatal cases have been reported for some of these adverse reactions: cardiac/vascular: myocarditis, pericarditis, vasculitis; nervous system: meningitis, encephalitis, myelitis and demyelination, myasthenic syndrome/myasthenia gravis (including exacerbation), Guillain-Barré syndrome, nerve paresis, autoimmune neuropathy; ocular: uveitis, iritis, and other ocular inflammatory toxicities can occur; gastrointestinal: pancreatitis to include increases in serum amylase and lipase levels, gastritis, duodenitis; musculoskeletal and connective tissue: myositis/polymyositis, rhabdomyolysis, and associated sequelae including renal failure, arthritis, polymyalgia rheumatica; endocrine: hypoparathyroidism; other (hematologic/immune): hemolytic anemia, aplastic anemia, hemophagocytic lymphohistiocytosis (HLH), systemic inflammatory response syndrome, histiocytic necrotizing lymphadenitis (Kikuchi lymphadenitis), sarcoidosis, immune thrombocytopenic purpura, solid organ transplant rejection.
• Some ocular IMAR cases can be associated with retinal detachment. Various grades of visual impairment, including blindness, can occur. If uveitis occurs in combination with other immune-mediated adverse reactions, consider a Vogt-Koyanagi-Harada–like syndrome, which has been observed in patients receiving OPDIVO, as this may require treatment with systemic corticosteroids to reduce the risk of permanent vision loss.
Infusion-Related Reactions
• OPDIVO can cause severe infusion-related reactions. Discontinue OPDIVO in patients with severe (Grade 3) or life-threatening (Grade 4) infusion-related reactions. Interrupt or slow the rate of infusion in patients with mild (Grade 1) or moderate (Grade 2) infusion-related reactions. In patients receiving OPDIVO monotherapy as a 60-minute infusion, infusion-related reactions occurred in 6.4% (127/1994) of patients. In a separate trial in which patients received OPDIVO monotherapy as a 60-minute infusion or a 30-minute infusion, infusion-related reactions occurred in 2.2% (8/368) and 2.7% (10/369) of patients, respectively. Additionally, 0.5% (2/368) and 1.4% (5/369) of patients, respectively, experienced adverse reactions within 48 hours of infusion that led to dose delay, permanent discontinuation or withholding of OPDIVO.
Complications of Allogeneic Hematopoietic Stem Cell Transplantation
• Fatal and other serious complications can occur in patients who receive allogeneic hematopoietic stem cell transplantation (HSCT) before or after being treated with OPDIVO. Transplant-related complications include hyperacute graft-versus-host-disease (GVHD), acute GVHD, chronic GVHD, hepatic veno-occlusive disease (VOD) after reduced intensity conditioning, and steroid-requiring febrile syndrome (without an identified infectious cause). These complications may occur despite intervening therapy between OPDIVO and allogeneic HSCT.
• Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefit versus risks of treatment with OPDIVO prior to or after an allogeneic HSCT.
Embryo-Fetal Toxicity
• Based on its mechanism of action and findings from animal studies, OPDIVO can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with OPDIVO and for at least 5 months after the last dose.
Increased Mortality in Patients with Multiple Myeloma when OPDIVO is Added to a Thalidomide Analogue and Dexamethasone
• In randomized clinical trials in patients with multiple myeloma, the addition of OPDIVO to a thalidomide analogue plus dexamethasone resulted in increased mortality. Treatment of patients with multiple myeloma with a PD-1 or PD-L1 blocking antibody in combination with a thalidomide analogue plus dexamethasone is not recommended outside of controlled clinical trials.
Lactation
• There are no data on the presence of OPDIVO in human milk, the effects on the breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment and for 5 months after the last dose.
Serious Adverse Reactions
• In Checkmate 649, serious adverse reactions occurred in 52% of patients treated with OPDIVO in combination with chemotherapy (n=782). The most frequent serious adverse reactions reported in ≥2% of patients treated with OPDIVO in combination with chemotherapy were vomiting (3.7%), pneumonia (3.6%), anemia (3.6%), pyrexia (2.8%), diarrhea (2.7%), febrile neutropenia (2.6%), and pneumonitis (2.4%). Fatal adverse reactions occurred in 16 (2.0%) patients who were treated with OPDIVO in combination with chemotherapy; these included pneumonitis (4 patients), febrile neutropenia (2 patients), stroke (2 patients), gastrointestinal toxicity, intestinal mucositis, septic shock, pneumonia, infection, gastrointestinal bleeding, mesenteric vessel thrombosis, and disseminated intravascular coagulation.
Common Adverse Reactions
• In Checkmate 649, the most common adverse reactions (≥20%) in patients treated with OPDIVO in combination with chemotherapy (n=782) were peripheral neuropathy (53%), nausea (48%), fatigue (44%), diarrhea (39%), vomiting (31%), decreased appetite (29%), abdominal pain (27%), constipation (25%), and musculoskeletal pain (20%).
Please see US Full Prescribing Information for OPDIVO.
References:
1. OPDIVO [package insert]. Princeton, NJ: Bristol-Myers Squibb Company.
2. Shitara K, Moehler M, Ajani JA, et al. Nivolumab plus chemotherapy vs chemotherapy as first-line treatment for advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma: 4-year follow-up of the CheckMate 649 study. Oral presentation at ASCO GI 2024. Abstract 306.
3. Mantziari S, St Amour P, Abboretti F, et al. A comprehensive review of prognostic factors in patients with gastric adenocarcinoma. Cancers (Basel). 2023;15(5):1628.
4. Imamura Y, Watanabe M, Oki E, Morita M, Baba H. Esophagogastric junction adenocarcinoma shares characteristics with gastric adenocarcinoma: literature review and retrospective multicenter cohort study. Ann Gastroenterol Surg. 2020;5(1):46-59.
5. Rogers MP, DeSantis AJ, DuCoin CG. Oligometastatic adenocarcinoma of the esophagus: current understanding, diagnosis, and therapeutic strategies. Cancers (Basel). 2021;13(17):4352.
6. Paydary K, Reizine N, Catenacci DVT. Immune-checkpoint inhibition in the treatment of gastro-esophageal cancer: a closer look at the emerging evidence. Cancers (Basel). 2021;13(23):5929.
7. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249.
8. Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27-40.
9. Janjigian YY, Ajani JA, Moehler M, et al. Nivolumab plus chemotherapy or ipilimumab vs chemotherapy as first-line treatment for advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma: CheckMate 649 study. Oral presentation at ESMO 2021. Abstract LBA7.
10. Shankaran V, Xiao H, Bertwistle D, et al. A comparison of real-world treatment patterns and clinical outcomes in patients receiving first-line therapy for unresectable advanced gastric or gastroesophageal junction cancer versus esophageal adenocarcinomas. Adv Ther. 2021;38:
707-720.
11. BMS-REF-NIVO-0256. Princeton, NJ: Bristol-Myers Squibb Company; 2024.
© 2024 Bristol-Myers Squibb Company. OPDIVO® is a registered trademark of Bristol-Myers Squibb Company.
1506-US-2300555 01/24

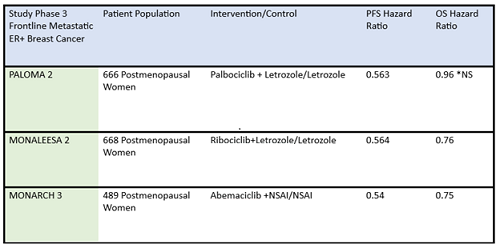
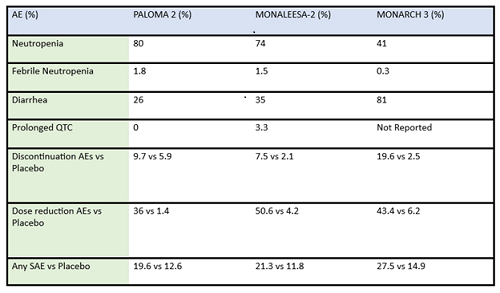


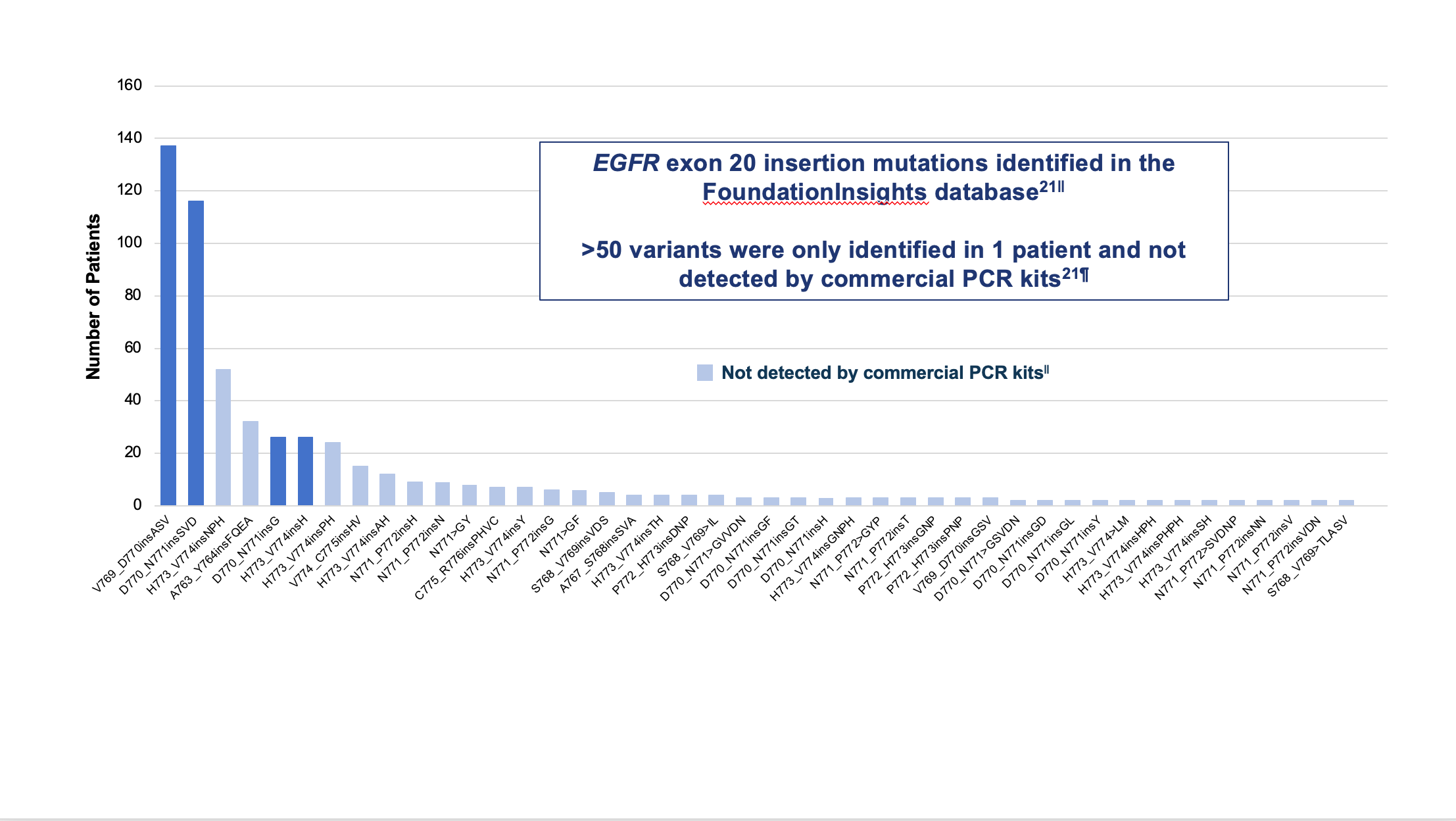
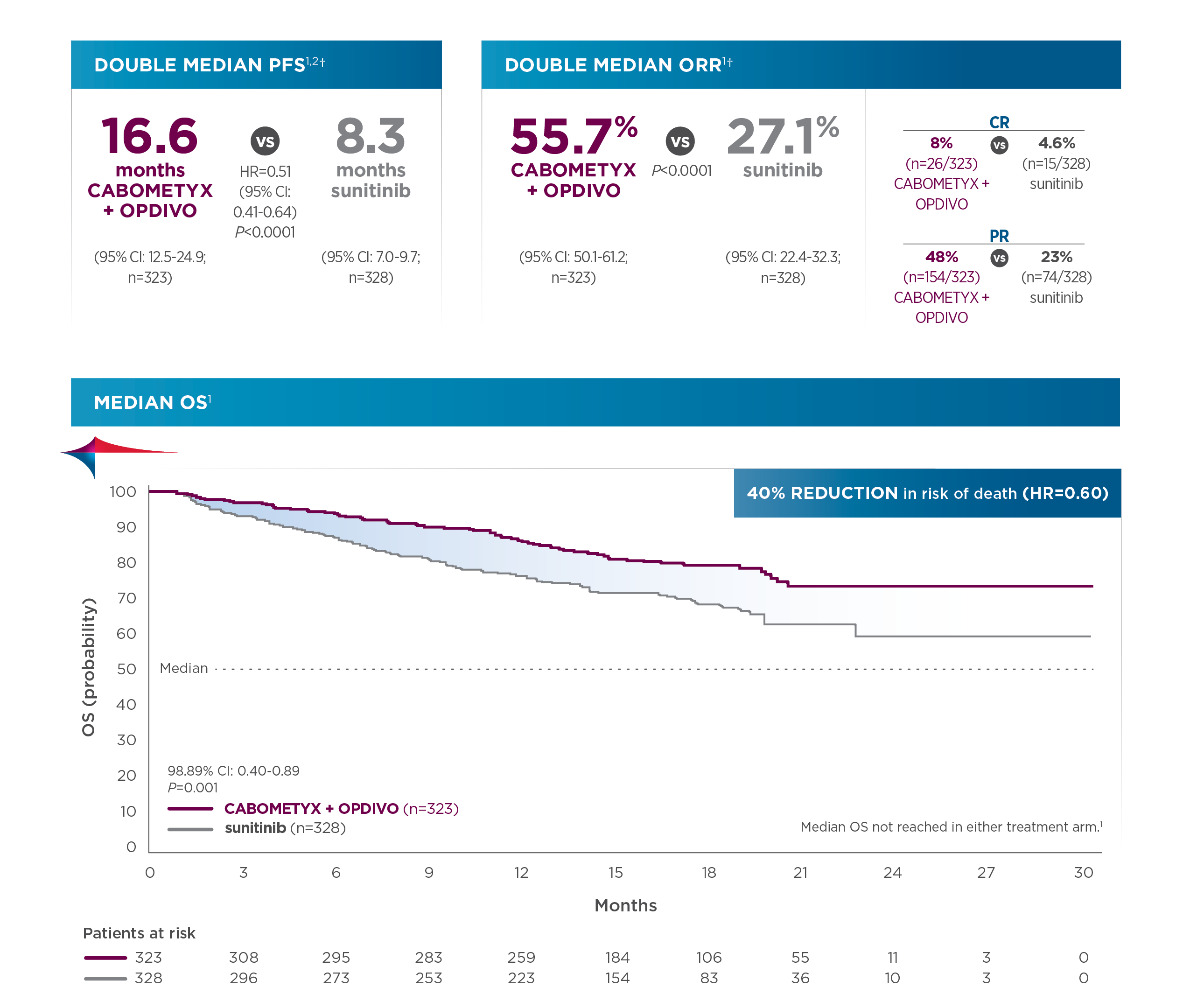
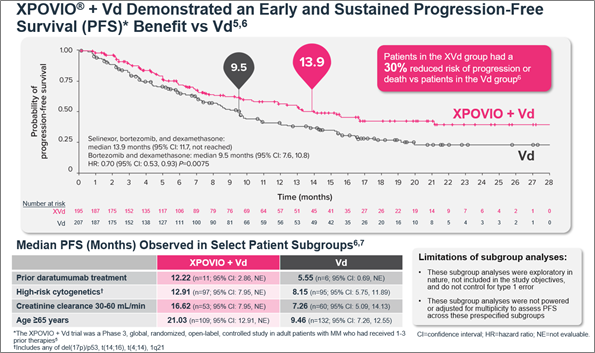 Figure 1. Median PFS in the XVd and Vd treatment groups (primary endpoint) and in select patient subgroups in the XVd trial.
Figure 1. Median PFS in the XVd and Vd treatment groups (primary endpoint) and in select patient subgroups in the XVd trial.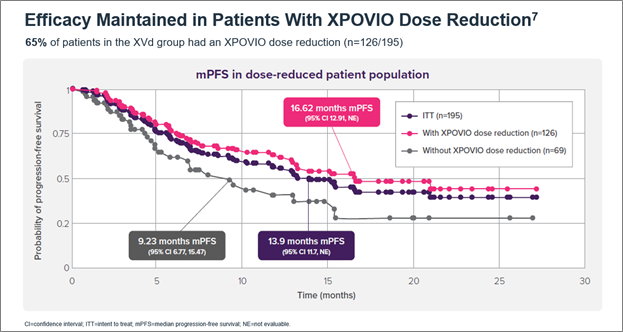 Figure 2. Median PFS in XPOVIO dose-reduced patients in the XVd trial.
Figure 2. Median PFS in XPOVIO dose-reduced patients in the XVd trial.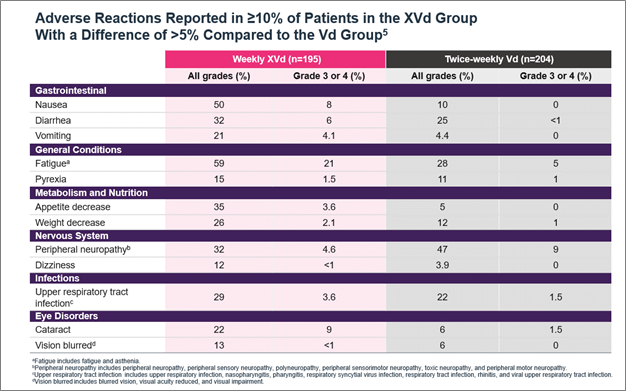 Figure 3. Adverse reactions reported in the XVd trial.
Figure 3. Adverse reactions reported in the XVd trial.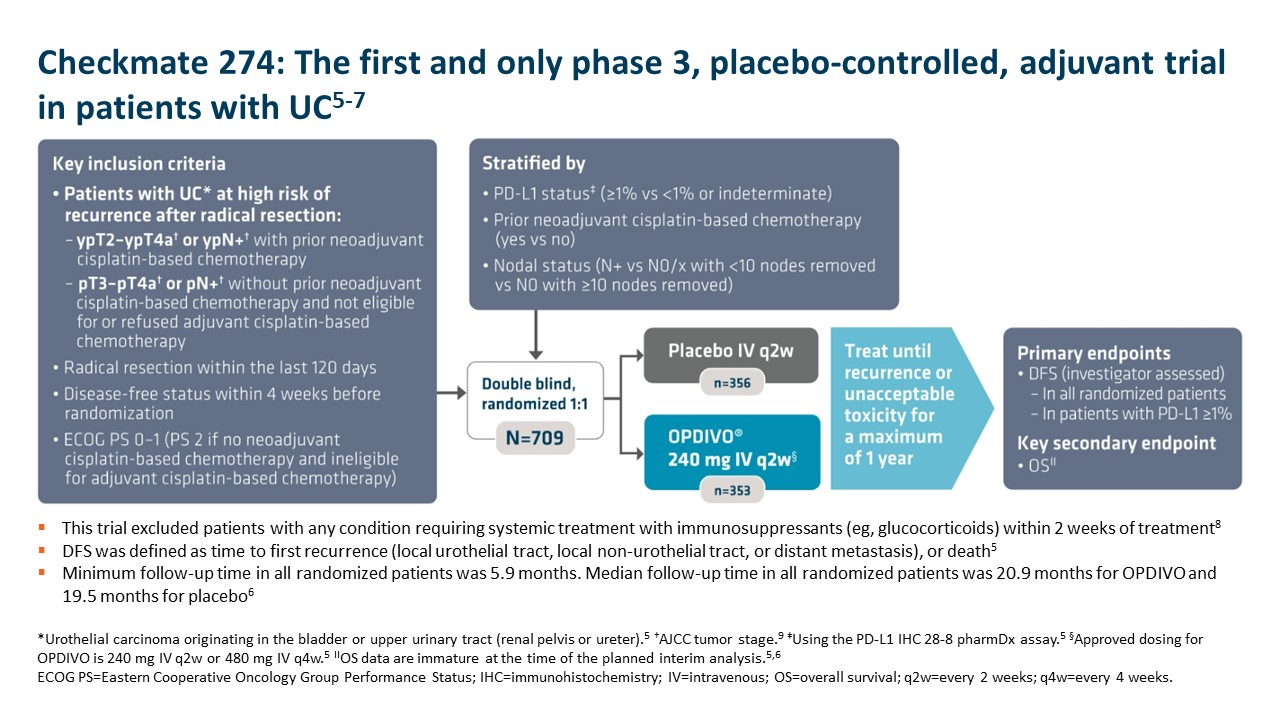 Important Safety Information
Important Safety Information