SUMMARY: Anemia is a common finding in patients with MyeloDysplastic Syndromes (MDS) and Beta-Thalassemia. These patients are in chronic need for transfusions which in turn can result in iron overload. Erythropoiesis Stimulating Agents (ESAs) are first-line therapy for anemia associated with lower-risk non-del(5q) MDS. ESAs such as Darbepoetin alfa and Epoetin alfa are re-engineered and recombinant DNA technology products of Erythropoietin (EPO), and they stimulate erythropoiesis by binding and activating the EPO receptor. There are however few treatment options for patients who are refractory to, unresponsive to, or ineligible for ESAs. There is therefore an unmet clinical need for safe and effective treatment options, to reduce the RBC transfusion burden in these patients. Beta-Thalassemia is an inherited hemoglobinopathy associated with an erythroid maturation defect and is characterized by ineffective erythropoiesis and impaired RBC maturation.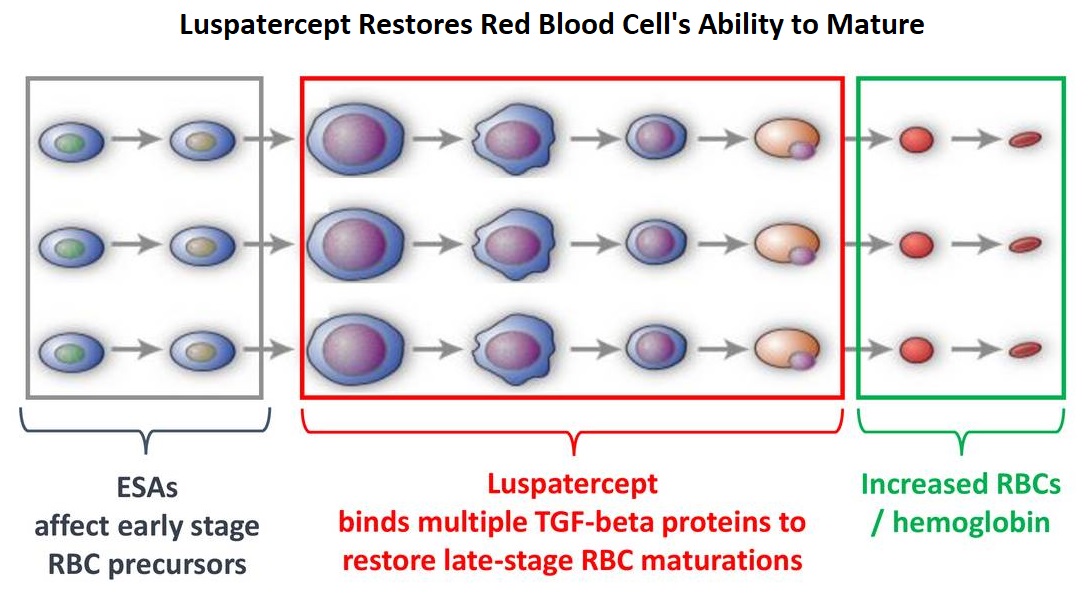
Luspatercept is a soluble fusion protein and is first-in-class erythroid maturation agent that enhances erythropoiesis by promoting late-stage Red Blood Cell precursor differentiation and maturation. It targets select Transforming Growth Factor (TGF)-β superfamily ligands such as GDF11, that regulate late-stage erythropoiesis. This results in a reduction in aberrant Smad2/3 signaling thereby promoting late-stage Red Blood Cell precursor differentiation and maturation. The following two, separate phase III studies have shown reduced blood transfusions requirements in two separate patient populations.
The MEDALIST trial is a randomized, double-blind, placebo-controlled phase III study which evaluated the efficacy and safety of Luspatercept in patients with anemia secondary to MDS, defined as very low-risk, low-risk, or Intermediate-risk with Ring Sideroblasts, according to the Revised International Prognostic Scoring System. Eligible patients were refractory, intolerant, or ineligible to receive ESAs and required RBC transfusions. A total of 229 patients (N=229) were randomized 2:1 to receive either Luspatercept at a starting dose level of 1mg/kg SC with titration up to 1.75 mg/kg if needed (N=153), or placebo SC (N=76), every 3 weeks for 24 weeks or more. The median age was 71 years and median time from diagnosis was 41.8 months. Approximately 95% of patients had previously received ESAs and 90% had an SF3B1 mutation. The Primary endpoint was RBC transfusion independence for 8 weeks or more between week 1 and 24. A key secondary endpoint was RBC transfusion independence for 12 weeks or more between week 1 and 24.
Among those receiving Luspatercept, 38% achieved the Primary endpoint of RBC transfusion independence for 8 weeks or more compared with 13.2% receiving placebo (P<0.0001). Further among those receiving Luspatercept, 28.1% achieved the key secondary endpoint of RBC transfusion independence for 12 weeks or more compared with 7.9% receiving placebo (P=0.0002). Additionally, patients receiving Luspatercept were more likely to achieve an mHI-E (modified hematologic improvement-erythroid) response, defined as a reduction in transfusion of 4 or more RBC units per 8 weeks or a mean hemoglobin increase of 1.5 g/dL or more per 8 weeks in the absence of transfusions, compared with patients receiving placebo (52.9% versus 11.8% during weeks 1-24; P<0.0001).
It was concluded that treatment with Luspatercept significantly decreased transfusion requirements among patients with low or Intermediate-risk MDS with Ring Sideroblasts.
The BELIEVE trial is a randomized, double-blind, placebo-controlled phase III study conducted to determine the efficacy and safety of Luspatercept in adult Beta-Thalassemia patients requiring regular RBC transfusions. In this study, 336 patients with Beta-Thalassemia or Hemoglobin E/ Beta-Thalassemia were randomized in a 2:1 to receive Luspatercept, at a starting dose of 1mg/kg with titration up to 1.25 mg/kg, or placebo, SC every 3 weeks for 48 weeks or more. Patients in both treatment groups continued to receive RBC transfusions and iron chelation therapy to maintain the same baseline Hgb level. Enrolled patients were 18 years or older and required regular RBC transfusions of 6-20 units in the 24 weeks prior to randomization with no transfusion-free period 35 days or more during that time. The median age was 30 years and 58% of patients were female. Patients received a median of 6 RBC units in the 12 weeks prior to treatment and 58% of patients in each treatment group had undergone splenectomy. The Primary endpoint was a 33% or more reduction in transfusion burden (with a reduction of 2 or more RBC units) during weeks 13–24, when compared with a 12-week baseline period.
It was noted that 21.4% of patients in the Luspatercept group achieved the Primary endpoint compared with 4.5% patients in the placebo group (P<0.0001). Towards the end of the trial, 20% of patients overall had decreased their transfusion units by one third or more, and 10% of patients had decreased their transfusions units by half or more. Overall, 70.5% of patients receiving Luspatercept achieved a 33% or more RBC transfusion reduction over any consecutive 12 weeks compared with 29.5% patients receiving placebo (P<0.0001).
It was concluded that treatment with Luspatercept resulted in significant reductions in RBC transfusion requirement, in adults with transfusion-dependent Beta-Thalassemia.
The most common adverse events included fatigue and muscle pain. It remains to be seen if Luspatercept would have similar efficacy in patients with high-risk MDS and patients with lower-risk MDS without ring sideroblasts.
The Medalist Trial: results of a phase 3, randomized, double-blind, placebo-controlled study of luspatercept to treat anemia in patients with very low-, low-, or intermediate-risk myelodysplastic syndromes (MDS) with ring sideroblasts (RS) who require red blood cell (RBC) transfusion. Fenaux P, Platzbecker U, Mufti GJ, et al. Presented at: 2018 ASH Annual Meeting; Dec. 1-4, 2018; San Diego. Abstract 1. https://ash.confex.com/ash/2018/webprogram/Paper110805.html
The Believe Trial: Results of a Phase 3, Randomized, Double-Blind, Placebo-Controlled Study of Luspatercept in Adult Beta-Thalassemia Patients Who Require Regular Red Blood Cell (RBC) Transfusions. Cappellini MD, Viprakasit V, Taher A, et al. Presented at: 2018 ASH Annual Meeting; Dec. 1-4, 2018; San Diego. Abstract 163. https://ash.confex.com/ash/2018/webprogram/Paper112435.html

