SUMMARY: It is estimated that in the US approximately 13,000 people are diagnosed with MyeloDysplastic Syndromes (MDS) each year. The prevalence has been estimated to be from 60,000 to 170,000 in the US. MyeloDysplastic Syndromes are a heterogenous group of stem cell disorders characterized by marrow failure resulting in cytopenias, mainly symptomatic anemia, with associated cytogenetic abnormalities, and abnormal cellular maturation with morphologic changes in clonal cells. Majority of the individuals diagnosed with MDS are 65 years or older and die as a result of infection and/or bleeding, consequent to bone marrow failure. About a third of patients with MDS develop Acute Myeloid Leukemia (AML).
Patients with Lower-risk MDS (Revised IPSS-Very Low, Low, or Intermediate risk ) often present with symptomatic anemia and these patients are in chronic need for RBC transfusions which in turn can result in iron overload and can have a negative impact on quality of life and Overall Survival. These patients are treated with Erythropoiesis Stimulating Agents (ESAs) as first line therapy. ESAs such as Darbepoetin alfa and Epoetin alfa are re-engineered and recombinant DNA technology products of Erythropoietin (EPO), and they stimulate erythropoiesis by binding and activating the EPO receptor. However, transfusion-dependent patients with serum EPO levels above 200 U per liter are less likely to respond to ESAs. Additionally, patients with MDS with ring sideroblasts have a shorter median duration of response to ESAs, than those who do not have ring sideroblasts. Patients with Lower-risk MDS with chromosome 5q deletion (del 5q) who are transfusion dependent are treated with Lenalidomide, regardless of previous treatment with ESAs. In contrast, only 39% of patients with non-del(5q) Lower-risk MDS receive second line therapy besides RBC transfusions, and there are few treatment options for patients who are refractory to, unresponsive to, or ineligible for ESAs. There is therefore an unmet clinical need for safe and effective treatment options, to reduce the RBC transfusion burden in these patients.
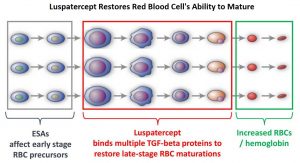
Signaling by the SMAD2 and SMAD3 pathway exerts an inhibitory effect on red cell maturation. This pathway is constitutively activated in the bone marrow cells of patients with MDS and diseases associated with ineffective erythropoiesis such as β-thalassemia. Luspatercept (REBLOZYL®) is a recombinant soluble fusion protein and is first-in-class erythroid maturation agent that enhances erythropoiesis by promoting late-stage Red Blood Cell precursor differentiation and maturation. It targets select Transforming Growth Factor (TGF)-β superfamily ligands such as GDF11, that regulate late-stage erythropoiesis. This results in a reduction in aberrant SMAD2 and SMAD3 signaling, thereby promoting late-stage RBC precursor differentiation and maturation. In the MEDALIST trial, Luspatercept significantly reduced the severity of anemia in patients with Lower-risk MDS with Ring Sideroblasts, who had been RBC transfusion-dependent, and who had disease that was refractory to, or unlikely to respond to ESAs.
COMMANDS trial is a global, randomized, controlled, open-label Phase III study, conducted to evaluate the efficacy and safety of Luspatercept in ESA-naive patients with Low Risk-MDS. This study included 354 eligible patients with lower-risk MDS as defined by the revised International Prognostic Scoring System (IPSS-R) criteria, with or without Ringed Sideroblasts. These patients had less than 5% bone marrow blasts and serum EPO levels less than 500 U/L and required red blood cell transfusions (defined as 2–6 RBC units/8 weeks for at least 8 weeks immediately prior to randomization). Patients were randomized 1:1 to receive Luspatercept (starting dose 1.0 mg/kg, titration up to 1.75 mg/kg) subcutaneous, once every 3 weeks for at least 24 weeks (N=178) or Epoetin alfa (starting dose 450 IU/kg, titration up to 1050 IU/kg, with a maximum permitted total dose of 80 000 IU)) weekly for at least 24 weeks (N=176). The median patient age was 74 years and both treatment groups were well balanced. Patients were stratified by baseline RBC transfusion burden (less than 4 units per 8 weeks versus 4 or more units per 8 weeks), endogenous serum EPO concentration (200 U/L or less versus more than 200 to less than 500 U/L), and Ring Sideroblast status (positive versus negative). The Primary endpoint was RBC independence for at least 12 weeks with a concurrent mean hemoglobin increase of at least 1.5 g/dL within the first 24 weeks.
At the planned interim analysis of 301 patients, 147 patients in the Luspatercept group and 154 patients in the Epoetin alfa group completed 24 weeks of treatment or discontinued earlier and the median treatment duration was 41.6 weeks for Luspatercept and 27.0 weeks for Epoetin alfa. Luspatercept was superior in efficacy compared to Epoetin alfa. Among patients receiving Luspatercept at the planned interim analysis, 58.5% achieved RBC transfusion independence for at least a 12-week consecutive period within the first 24 weeks, along with an increase in hemoglobin of at least 1.5 g/dL compared to 31.2% of patients receiving Epoetin alpha (P<0.0001). Luspatercept was effective regardless of baseline serum EPO, red blood cell transfusion burden, SF3B1 mutation status, or Ring Sideroblast status. These results were similar when comparisons of baseline serum erythropoietin and baseline RBC transfusion burden in subgroups were made. However, responses were different based on ring sideroblast status. Approximately 65% of Ringed Sideroblast positive patients on Luspatercept achieved the Primary endpoint compared to 26% on Epoetin alfa. Among Ringed Sideroblast negative patients, slightly more met this end point on Epoetin alfa (46% versus 41% respectively). The Secondary endpoints in the study included Hematologic Improvement-Erythroid Response of 8 weeks or more and RBC transfusion independence at 24 weeks or more and at 12 weeks or more. Similar to the Primary endpoint, Luspatercept was more effective than Epoetin alfa across these Secondary endpoints.
It was concluded from this interim analysis that compared with Epoetin alfa, Luspatercept significantly improved RBC transfusion independence, and erythroid response, as well as duration of response, with no new toxiciites. The authors added that this innovative therapy with Luspatercept could represent a new standard of care for patients with RBC transfusion dependent Low Risk Myelodysplastic Syndromes. Long-term follow up will be needed to confirm these results and further refine findings in patients with Lower-Risk Myelodysplastic Syndromes, including non-mutated SF3B1 or Ring Sideroblast-negative subgroups.
Efficacy and safety of luspatercept versus epoetin alfa in erythropoiesis-stimulating agent-naive, transfusion-dependent, lower-risk myelodysplastic syndromes (COMMANDS): interim analysis of a phase 3, open-label, randomised controlled trial. Platzbecker U, Della Porta MG, Santini V, et al. The Lancet. 2023; 402:373-385