SUMMARY: It is estimated that in the United States approximately 13,000 people are diagnosed with MyeloDysplastic Syndromes (MDS) each year. MyeloDysplastic Syndromes are a heterogenous group of stem cell disorders characterized by marrow failure resulting in cytopenias with associated cytogenetic abnormalities, and abnormal cellular maturation with morphologic changes in clonal cells. Majority of the individuals diagnosed with MDS are aged 65 years and older and die as a result of infection and/or bleeding consequent to bone marrow failure. About a third of patients with MDS develop Acute Myeloid Leukemia (AML).
Patients with Lower-risk MDS (Revised IPSS-Very Low, Low, or Intermediate risk ) often present with symptomatic anemia and these patients are in chronic need for RBC transfusions which in turn can result in iron overload and can have a negative impact on quality of life and Overall Survival. These patients are treated with Erythropoiesis Stimulating Agents (ESAs) as first line therapy. ESAs such as Darbepoetin alfa and Epoetin alfa are re-engineered and recombinant DNA technology products of Erythropoietin (EPO), and they stimulate erythropoiesis by binding and activating the EPO receptor. However, transfusion-dependent patients with serum EPO levels above 200 U per liter are less likely to respond to ESAs. Additionally, patients with MDS with ring sideroblasts have a shorter median duration of response to ESAs, than those who do not have ring sideroblasts. Patients with Lower-risk MDS with chromosome 5q deletion (del 5q) who are transfusion dependent are treated with Lenalidomide, regardless of previous treatment with ESAs. In contrast, only 39% of patients with non-del(5q) Lower-risk MDS receive second line therapy besides RBC transfusions, and there are few treatment options for patients who are refractory to, unresponsive to, or ineligible for ESAs. There is therefore an unmet clinical need for safe and effective treatment options, to reduce the RBC transfusion burden in these patients.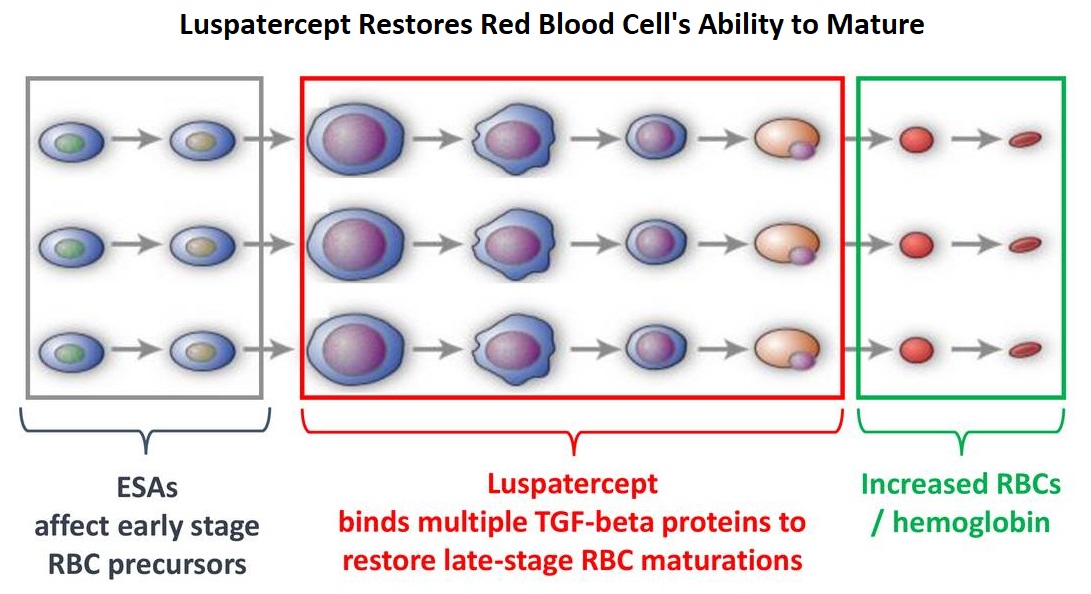
Signaling by the SMAD2 and SMAD3 pathway exerts an inhibitory effect on red cell maturation. This pathway is constitutively activated in the bone marrow cells of patients with MDS and diseases associated with ineffective erythropoiesis such as β-thalassemia. REBLOZYL® (Luspatercept) is a recombinant soluble fusion protein and is first-in-class erythroid maturation agent that enhances erythropoiesis by promoting late-stage Red Blood Cell precursor differentiation and maturation. It targets select Transforming Growth Factor (TGF)-β superfamily ligands such as GDF11, that regulate late-stage erythropoiesis. This results in a reduction in aberrant SMAD2 and SMAD3 signaling, thereby promoting late-stage RBC precursor differentiation and maturation. In a Phase II study, treatment of Lower-risk MDS patients with REBLOZYL® resulted in 38% of patients being transfusion independent for 8 weeks or longer and this benefit was even more so among patients with 15% or more ring sideroblasts.
The MEDALIST trial is a randomized, double-blind, placebo-controlled Phase III study which evaluated the efficacy and safety of REBLOZYL® in patients with anemia secondary to MDS, defined as Very Low-Risk, Low-Risk, or Intermediate-Risk with Ring Sideroblasts, according to the Revised International Prognostic Scoring System (R-IPSS). Eligible patients were refractory, intolerant, or ineligible to receive ESAs and required RBC transfusions. A total of 229 patients (N=229) were randomized 2:1 to receive either REBLOZYL® at a starting dose level of 1mg/kg SC with titration up to 1.75 mg/kg if needed (N=153), or placebo SC (N=76), every 3 weeks for 24 weeks or more. The median age was 71 years and median time from diagnosis was 41.8 months. Approximately 95% of patients had previously received ESAs and 90% had an SF3B1 mutation. The Primary endpoint was RBC transfusion independence for 8 weeks or more between week 1 and 24. A key Secondary endpoint was RBC transfusion independence for 12 weeks or more between week 1 and 24.
Among those receiving REBLOZYL®, 38% achieved the Primary endpoint of RBC transfusion independence for 8 weeks or more, compared with 13% receiving placebo (P<0.001). Further among those receiving REBLOZYL®, 28% achieved the key Secondary endpoint of RBC transfusion independence for 12 weeks or more compared with 8% receiving placebo (P<0.001). The median duration of the longest, single continuous period of response to REBLOZYL® was 30.6 weeks, and 13.6 weeks in the placebo group. Among patients who had a baseline transfusion burden of 4 to less than 6 units per 8 weeks, 37% of those in the REBLOZYL® group and 4% of those in the placebo group had a response. Additionally, patients receiving REBLOZYL® were more likely to achieve an mHI-E (modified Hematologic Improvement-Erythroid) response, (defined as a reduction in transfusion of 4 or more RBC units per 8 weeks or a mean hemoglobin increase of 1.5 g/dL or more per 8 weeks, in the absence of transfusions), compared with patients receiving placebo (53% versus 12% during weeks 1-24; P<0.0001). A mean increase in hemoglobin level of at least 1 g/dL during weeks 1 to 24 was noted in 35% of patients who received REBLOZYL® and in 8% of patients who received placebo. The most common adverse events of any grade associated with REBLOZYL® included fatigue, diarrhea, asthenia, nausea and dizziness, and the incidence of adverse events decreased over time.
It was concluded that treatment with REBLOZYL® significantly reduced the severity of anemia in patients with Lower-risk MDS with ring sideroblasts, who had been RBC transfusion-dependent, and who had disease that was refractory to or unlikely to respond to ESAs. Luspatercept in Patients with Lower-Risk Myelodysplastic Syndromes. Fenaux P, Platzbecker U, Mufti GJ, et al. N Engl J Med 2020; 382:140-151
Tag: Myelodysplastic Syndrome
Telomerase Inhibitor Imetelstat Provides Durable Transfusion Independence in Myelodysplastic Syndrome
SUMMARY: It is estimated that in the United States approximately 13,000 people are diagnosed with MyeloDysplastic Syndromes (MDS) each year. MyeloDysplastic Syndromes are a heterogenous group of stem cell disorders characterized by marrow failure resulting in cytopenias with associated cytogenetic abnormalities, and abnormal cellular maturation with morphologic changes in clonal cells. Majority of the individuals diagnosed with MDS are aged 65 years and older and die as a result of infection and/or bleeding, consequent to bone marrow failure. About a third of patients with MDS develop Acute Myeloid Leukemia (AML). Patients with low-risk MDS have an indolent disease course with a median survival of about 6 years with no therapeutic intervention. Patients with intermediate and higher-risk disease however have a shorter median survival even with treatment, with approximately a third of the patients progressing to AML within 3 years. The International Prognostic Scoring System (IPSS) for MDS has 4 risk groups based on Total Risk Score (Low, Intermediate-1, Intermediate-2 and High). The three prognostic factors scored to predict the course of the patient's disease include, percentage of blast cells in the bone marrow, type of chromosomal changes in the marrow cells and number of cytopenias (anemia, neutropenia or thrombocytopenia).
Management of patients with MDS includes supportive care with Erythropoiesis Stimulating Agents (ESAs), hypomethylating agents such as VIDAZA® (Azacitidine) and DACOGEN® (Decitabine), Immunomodulatory agents such as REVLIMID® (Lenalidomide), and Immunosuppressive agents such as AntiThymocyte Globulin (ATG) and Cyclosporine. Symptomatic patients with MDS are often treated with either VIDAZA® or DACOGEN® as these agents have been shown to improve survival in higher-risk MDS patients. It has remained unclear however, if one is better than the other.
Erythropoiesis Stimulating Agents (ESAs) are first-line therapy for anemia associated with lower-risk non-del(5q) MDS. ESAs such as Darbepoetin alfa and Epoetin alfa are re-engineered and recombinant DNA technology products of Erythropoietin (EPO), and they stimulate erythropoiesis by binding and activating the EPO receptor. There are however limited treatment options for RBC Transfusion Dependent (TD), Low Risk (IPSS Low/Int-1) MDS patients, who are Relapsed/Refractory to ESAs. There is therefore an unmet clinical need for safe and effective treatment options, to reduce the RBC transfusion burden in these patients. Imetelstat is a first-in-class Telomerase inhibitor that targets cells with short telomere length and active Telomerase, a feature often observed in some MDS patients across all stages of their disease. Higher Telomerase activity and shorter Telomeres in the blood cells of some patients with lower-risk MDS are known to predict for shorter overall survival.
IMerge is an ongoing global Phase II/III study of Imetelstat in RBC Transfusion Dependent patients with Low Risk-MDS (IPSS Low or Int-1). Previously reported data have demonstrated clinical benefit with Imetelstat in Low Risk-MDS patients inducing durable Transfusion Independence (Steensma et al ASH 2018 Abstr463). The authors now reported updated efficacy data in Low Risk, non-del(5q) MDS patients, Relapsed/Refractory to ESAs and Lenalidomide/HypoMethylating Agents naïve, from the open-label, single-arm Part 1 of IMerge study.
Part 1 of the IMerge study included patients with Low Risk MDS, who were heavily transfused (4 or more units /8wks), were Refractory or Relapsed on ESA or had serum EPO level of more than 500 mU/mL. This part of the study included 38 patients who were non-del(5q) and had not received either Lenalidomide/HypoMethylating Agents. Imetelstat was administered at 7.5 mg/kg IV every 4 weeks. The median patient age was 72 years, median baseline RBC transfusion burden was 8U/8weeks (range 4-14), 37% of the patients had IPSS Intermediate-1 risk score, 71% had WHO 2001 classification RARS (Refractory Anemia with Ringed Sideroblasts) or RCMD-RS (Refractory Cytopenia with Multilineage Dysplasia and Ringed Sideroblast) subtype and 32% with evaluable serum EPO levels had baseline level of more than 500 mU/mL. The Primary endpoint was 8-week Transfusion Independence rate. Secondary endpoints included 24-week Transfusion Independence rate, Safety, Duration of Transfusion Independence, and Hematologic Improvement rate. The median follow up was 12.1 months. The authors in this publication reported long-term efficacy, safety and biomarker data from these 38 patients.
Treatment with single-agent Imetelstat resulted in 8-week Transfusion Independence rate of 45% and the median Transfusion Independence duration was 8.5 months. Among those responding to Imetelstat, 59% remained transfusion free for over 24 weeks. The presence of Ring Sideroblasts or baseline serum EPO levels did not have an impact on 8-week Transfusion Independence rate. The 24-week Transfusion Independence rate was 26%. Erythroid Hematologic Improvement, defined as transfusion reduction by at least 4 units/8 weeks (IWG2006), was achieved in 68% of the patients. All patients (N=6) who had IPSS- Intermediate/poor cytogenetic risk achieved 8-week Transfusion Independence and 2 patients achieved partial cytogenetic response. Post treatment decrease in Telomerase (human Telomerase Reverse Transcriptase-hTERT) RNA level was observed in 73.5% of patients. Further, among patients with pre and post-treatment mutation analyses, six patients had SF3B1 mutations at baseline, and 2 patients who had a decrease in the mutation burden had longest Transfusion Independence duration on study. The most frequently reported adverse events were manageable and reversible grade 3 cytopenias.
It was concluded that treatment with single agent Imetelstat resulted in meaningful and durable Transfusion Independence, in Transfusion Dependent patients with non-del(5q) Lower-Risk MDS, who had relapsed or were refractory to ESA. Transfusion Independence was observed across different clinical subgroups, including patients with Intermediate and Poor cytogenetic risk, with a positive effect on malignant mutant clones. TREATMENT WITH IMETELSTAT PROVIDES DURABLE TRANSFUSION INDEPENDENCE (TI) IN HEAVILY TRANSFUSED NON-DEL(5Q) LOWER RISK MDS (LR-MDS) RELAPSED/REFRACTORY (R/R) TO ERYTHROPOIESIS STIMULATING AGENTS (ESAS). Fenaux P, Steensma DP, Eygen KV, et al. Presentation during European Hematology Association, Jun 15, 2019; 267420; S837
Luspatercept Reduces Blood Transfusion Requirements in MDS and Beta-Thalassemia
SUMMARY: Anemia is a common finding in patients with MyeloDysplastic Syndromes (MDS) and Beta-Thalassemia. These patients are in chronic need for transfusions which in turn can result in iron overload. Erythropoiesis Stimulating Agents (ESAs) are first-line therapy for anemia associated with lower-risk non-del(5q) MDS. ESAs such as Darbepoetin alfa and Epoetin alfa are re-engineered and recombinant DNA technology products of Erythropoietin (EPO), and they stimulate erythropoiesis by binding and activating the EPO receptor. There are however few treatment options for patients who are refractory to, unresponsive to, or ineligible for ESAs. There is therefore an unmet clinical need for safe and effective treatment options, to reduce the RBC transfusion burden in these patients. Beta-Thalassemia is an inherited hemoglobinopathy associated with an erythroid maturation defect and is characterized by ineffective erythropoiesis and impaired RBC maturation.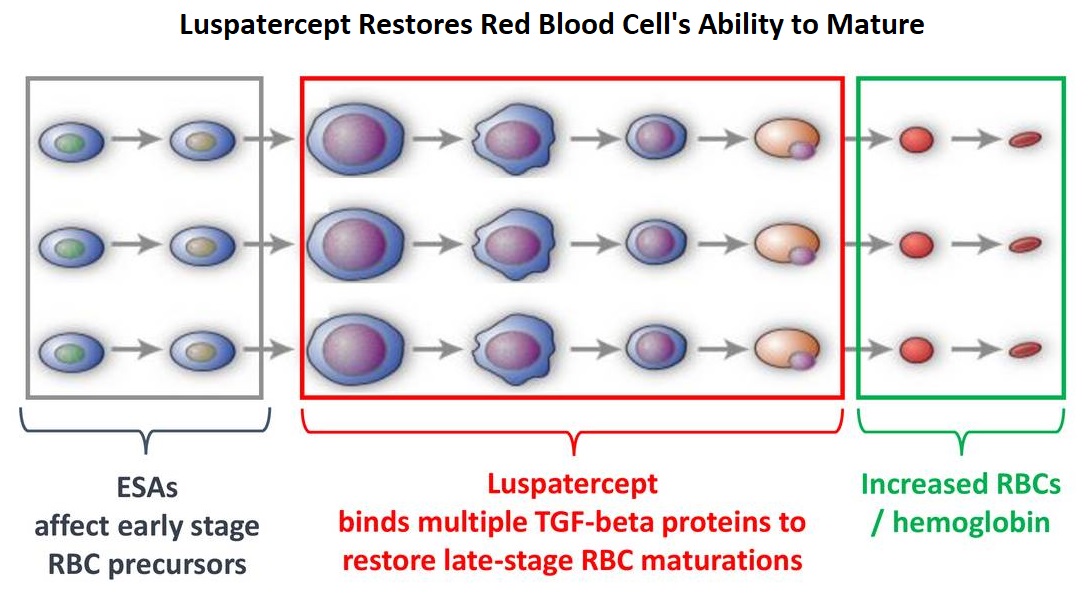
Luspatercept is a soluble fusion protein and is first-in-class erythroid maturation agent that enhances erythropoiesis by promoting late-stage Red Blood Cell precursor differentiation and maturation. It targets select Transforming Growth Factor (TGF)-β superfamily ligands such as GDF11, that regulate late-stage erythropoiesis. This results in a reduction in aberrant Smad2/3 signaling thereby promoting late-stage Red Blood Cell precursor differentiation and maturation. The following two, separate phase III studies have shown reduced blood transfusions requirements in two separate patient populations.
The MEDALIST trial is a randomized, double-blind, placebo-controlled phase III study which evaluated the efficacy and safety of Luspatercept in patients with anemia secondary to MDS, defined as very low-risk, low-risk, or Intermediate-risk with Ring Sideroblasts, according to the Revised International Prognostic Scoring System. Eligible patients were refractory, intolerant, or ineligible to receive ESAs and required RBC transfusions. A total of 229 patients (N=229) were randomized 2:1 to receive either Luspatercept at a starting dose level of 1mg/kg SC with titration up to 1.75 mg/kg if needed (N=153), or placebo SC (N=76), every 3 weeks for 24 weeks or more. The median age was 71 years and median time from diagnosis was 41.8 months. Approximately 95% of patients had previously received ESAs and 90% had an SF3B1 mutation. The Primary endpoint was RBC transfusion independence for 8 weeks or more between week 1 and 24. A key secondary endpoint was RBC transfusion independence for 12 weeks or more between week 1 and 24.
Among those receiving Luspatercept, 38% achieved the Primary endpoint of RBC transfusion independence for 8 weeks or more compared with 13.2% receiving placebo (P<0.0001). Further among those receiving Luspatercept, 28.1% achieved the key secondary endpoint of RBC transfusion independence for 12 weeks or more compared with 7.9% receiving placebo (P=0.0002). Additionally, patients receiving Luspatercept were more likely to achieve an mHI-E (modified hematologic improvement-erythroid) response, defined as a reduction in transfusion of 4 or more RBC units per 8 weeks or a mean hemoglobin increase of 1.5 g/dL or more per 8 weeks in the absence of transfusions, compared with patients receiving placebo (52.9% versus 11.8% during weeks 1-24; P<0.0001).
It was concluded that treatment with Luspatercept significantly decreased transfusion requirements among patients with low or Intermediate-risk MDS with Ring Sideroblasts.
The BELIEVE trial is a randomized, double-blind, placebo-controlled phase III study conducted to determine the efficacy and safety of Luspatercept in adult Beta-Thalassemia patients requiring regular RBC transfusions. In this study, 336 patients with Beta-Thalassemia or Hemoglobin E/ Beta-Thalassemia were randomized in a 2:1 to receive Luspatercept, at a starting dose of 1mg/kg with titration up to 1.25 mg/kg, or placebo, SC every 3 weeks for 48 weeks or more. Patients in both treatment groups continued to receive RBC transfusions and iron chelation therapy to maintain the same baseline Hgb level. Enrolled patients were 18 years or older and required regular RBC transfusions of 6-20 units in the 24 weeks prior to randomization with no transfusion-free period 35 days or more during that time. The median age was 30 years and 58% of patients were female. Patients received a median of 6 RBC units in the 12 weeks prior to treatment and 58% of patients in each treatment group had undergone splenectomy. The Primary endpoint was a 33% or more reduction in transfusion burden (with a reduction of 2 or more RBC units) during weeks 13–24, when compared with a 12-week baseline period.
It was noted that 21.4% of patients in the Luspatercept group achieved the Primary endpoint compared with 4.5% patients in the placebo group (P<0.0001). Towards the end of the trial, 20% of patients overall had decreased their transfusion units by one third or more, and 10% of patients had decreased their transfusions units by half or more. Overall, 70.5% of patients receiving Luspatercept achieved a 33% or more RBC transfusion reduction over any consecutive 12 weeks compared with 29.5% patients receiving placebo (P<0.0001).
It was concluded that treatment with Luspatercept resulted in significant reductions in RBC transfusion requirement, in adults with transfusion-dependent Beta-Thalassemia.
The most common adverse events included fatigue and muscle pain. It remains to be seen if Luspatercept would have similar efficacy in patients with high-risk MDS and patients with lower-risk MDS without ring sideroblasts.
The Medalist Trial: results of a phase 3, randomized, double-blind, placebo-controlled study of luspatercept to treat anemia in patients with very low-, low-, or intermediate-risk myelodysplastic syndromes (MDS) with ring sideroblasts (RS) who require red blood cell (RBC) transfusion. Fenaux P, Platzbecker U, Mufti GJ, et al. Presented at: 2018 ASH Annual Meeting; Dec. 1-4, 2018; San Diego. Abstract 1. https://ash.confex.com/ash/2018/webprogram/Paper110805.html
The Believe Trial: Results of a Phase 3, Randomized, Double-Blind, Placebo-Controlled Study of Luspatercept in Adult Beta-Thalassemia Patients Who Require Regular Red Blood Cell (RBC) Transfusions. Cappellini MD, Viprakasit V, Taher A, et al. Presented at: 2018 ASH Annual Meeting; Dec. 1-4, 2018; San Diego. Abstract 163. https://ash.confex.com/ash/2018/webprogram/Paper112435.html
DACOGEN® May Be Superior to VIDAZA® in Higher-Risk MDS Patients
SUMMARY: It is estimated that in the United States approximately 13,000 people are diagnosed with MyeloDysplastic Syndromes (MDS) each year. MyeloDysplastic Syndromes are a heterogenous group of stem cell disorders characterized by marrow failure resulting in cytopenias with associated cytogenetic abnormalities, and abnormal cellular maturation with morphologic changes in clonal cells. Majority of the individuals diagnosed with MDS are aged 65 years and older and die as a result of infection and/or bleeding consequent to bone marrow failure. About a third of patients with MDS develop Acute Myeloid Leukemia (AML). Patients with low-risk MDS have an indolent disease course with a median survival of about 6 years with no therapeutic intervention. Patients with intermediate and higher-risk disease however have a shorter median survival even with treatment, with approximately a third of the patients progressing to AML within 3 years.
Management of patients with MDS includes supportive care with Erythropoiesis Stimulating Agents (ESA), hypomethylating agents such as VIDAZA® (Azacitidine) and DACOGEN® (Decitabine), immunomodulatory agents such as REVLIMID® (Lenalidomide), and immunosuppressive agents such as AntiThymocyte Globulin (ATG) and Cyclosporine. Symptomatic patients with MDS are often treated with either VIDAZA® or DACOGEN® as these agents have been shown to improve survival in higher-risk MDS patients. It has remained unclear however, if one is better than the other. 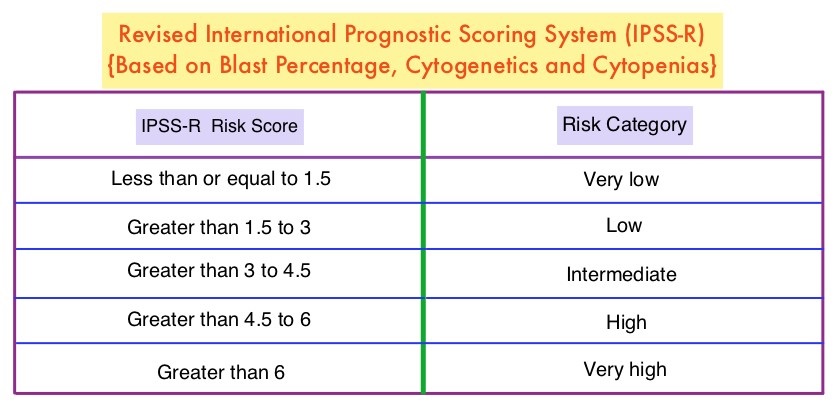
To address this question the authors conducted a phase II study, in which 113 patients with low (36%), intermediate (30%), and high (20%) – risk MDS, as determined by the Revised International Prognostic Scoring System (IPSS-R), were randomly assigned to receive either VIDAZA® 75 mg/m2 IV/SC daily (N=40) or DACOGEN® 20 mg/m2 IV daily (N=73), for 3 consecutive days, with the cycle repeated every 28 days. Patients received a median of 9 cycles. The primary endpoint was Overall Response Rate (ORR).
It was noted that the ORR was 70% and 49% for patients treated with DACOGEN® and VIDAZA® respectively (P=0.03). Cytogenetic response rates were 61% and 25% respectively (P=0.02). Thirty-two percent (32%) of patients treated with DACOGEN® became transfusion independent compared with 16% of patients treated with VIDAZA® Among patients with 5% or more bone marrow blasts, all responded to DACOGEN® whereas only 36% responded to VIDAZA® (P<0.001). With a median follow up of 20 months, the median Event Free Survival for patients treated with DACOGEN® was 20 months and 13 months for those treated with VIDAZA®, and these outcomes were negatively impacted by the presence of TP53 and ZRSR2 mutations. More patients in the DACOGEN® group experienced myelosuppression, and grade 3 toxicities were rare.
The authors concluded that lower doses of DACOGEN® and VIDAZA® are safe and effective in symptomatic patients with MDS, and DACOGEN® is more effective compared to VIDAZA®, in patients with higher-risk features. A randomized phase II study of low-dose decitabine versus low-dose azacitidine in lower risk MDS and MDS/MPN. Jabbour E, Short NJ, Montalban-Bravo G, et al. Blood. 2017 Aug 3. pii: blood-2017-06-788497. doi: 10.1182/blood-2017-06-788497. [Epub ahead of print]
Randomized Open-Label Phase II Study of Decitabine in Patients With Low- or Intermediate-Risk Myelodysplastic Syndromes
SUMMARY: The MyeloDysplastic Syndromes (MDS) are disorders of hematopoietic stem cells, characterized by one or more peripheral blood cytopenias. These syndromes may arise de novo or can be secondary, following treatment with chemotherapy and/or radiation therapy for other malignancies. It can also manifest after environmental exposures. The International Prognostic Scoring System (IPSS), based on the score, divides patients with MDS into Lower Risk (IPSS low and intermediate-1 scores) and Higher Risk (IPSS intermediate-2 and high scores) groups. This classification remains arbitrary because of the heterogeneity in the Lower Risk group and poor outcomes in certain Lower Risk subset of patients. DACOGEN® (Decitabine) exerts its antineoplastic effects by inhibitng DNA methyltransferase, resulting in hypomethylation of DNA and apoptosis. The authors in this Phase II study evaluated the outcomes in patients with Low or Intermediate-1 risk MDS using lower doses of DACOGEN® given subcutaneously on two different treatment schedules. Of the 67 randomized patients, 43 patients received DACOGEN® 20mg/m2 SC QD for three consecutive days every 28 days (Schedule A) or 20mg/m2 SC given on days 1, 8 and 15, of a 28 day cycle (Schedule B). Treatment was planned for up to one year. Primary endpoint was Overall Improvement Rate (OIR) and this included Complete Remission (CR), Partial Remission (PR), marrow CR or Hematologic Improvement (HI). Secondary end points included HI, transfusion independence, cytogenetic response, overall survival (OS), and time to acute myeloid leukemia or death. Taken as a group, there was no difference in the OIR (23%), HI, transfusion independence or cytogenetic response between schedule A and schedule B. However, patients on schedule A had more CR's making this a protocol defined superior schedule. Median OS was not reached. Transfusion independency was noted in 67% of the patient's on schedule A and 59% of the patient's on schedule B. Further, approximately 70% of the patients were alive at 500 days. The most frequent adverse events in schedule A and B were anemia (23% vs 18%), neutropenia (28% vs 36%) and thrombocytopenia (16% vs 32%). Based on these promising results, the authors recommended DACOGEN® given on three consecutive days SC every 28 days as per Schedule A, for patients with Low or Intermediate-1 risk MDS. Garcia-Manero G, Jabbour E, Borthakur G, et al. J Clin Oncol 2013;31:2548-2553
