SUMMARY: The FDA on January 9, 2020, approved AYVAKIT® (Avapritinib) for adults with unresectable or metastatic GastroIntestinal Stromal Tumor (GIST) harboring a Platelet-Derived Growth Factor Receptor Alpha (PDGFRA) Exon 18 mutation, including D842V mutations. The American Cancer Society estimates that in the US, about 4000-5000 cases of GastroIntestinal Stromal Tumors (GISTs) are diagnosed each year. GI Stromal Tumor (GIST) is one of the most common types of Soft Tissue Sarcoma and can develop anywhere along the GI tract, but are primarily found in the stomach (60%) and small intestine (30%). GISTs originate from the interstitial cells of Cajal or related stem cells and are associated with activating mutations in KIT-CD117 (80%) or PDGFRA (5-10%). These two mutations are mutually exclusive and are important in the molecular pathogenesis of these tumors. PDGFRA-mutated GISTs are mostly of gastric origin and display epithelioid morphology or mixed epithelioid and spindle histology. Gain-of-function mutations lead to constitutive, ligand independent activation of PDGFRA and its downstream pathways, subsequently resulting in cell proliferation and apotosis inhibition. Primary PDGFRA mutations are found mainly in Exons 12 and 18 and rarely in Exon 14. The most frequent mutation results in an Exon 18 D842V substitution, detected in up to 75% of all PDGFRA-mutated tumors 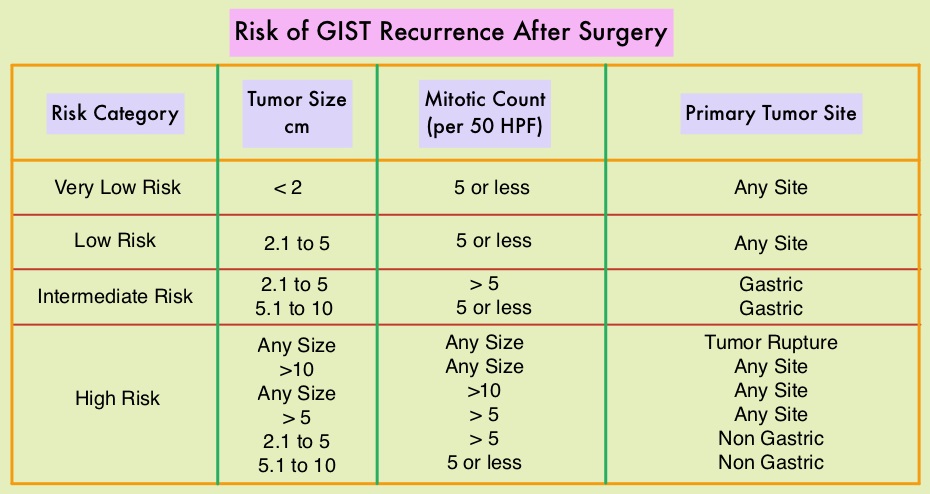
Most patients with GIST are diagnosed between 50 to 80 years of age, and patients may present with GI bleeding or as incidental findings during surgery or imaging, and occasionally with tumor rupture or bowel obstruction. Approximately two thirds of the patients with GISTs are cured with surgery but recurrences are frequent and this risk of relapse is dependent on the tumor size, mitotic rate and primary tumor site. The risk stratification of GISTs by Joensuu, unlike the NIH criteria, takes into account primary tumor site and tumor rupture as well, which can influence outcomes. Treatment of patients with advanced or metastatic GIST with Tyrosine Kinase Inhibitor GLEEVEC® (Imatinib) achieves high Objective Response and diseases stabilization rates. Patients with KIT Exon 9 mutation have a poor prognosis compared to those with KIT Exon 11 mutation (most common) and benefit from a higher dose of GLEEVEC® (800 mg daily). Following progression on GLEEVEC®, FDA approved therapies include SUTENT® (Sunitinib) and STIVARGA® (Regorafenib). Patients with PDGFRA D842V mutation are GLEEVEC® resistant.
AYVAKIT® is an oral, precision therapy, and is a potent and selective type 1 inhibitor of KIT and PDGFRA mutant kinases, and is also uniquely designed to selectively bind and inhibit D816V mutant KIT, the common driver of disease in approximately 95 percent of all Systemic Mastocytosis (SM) patients.
The present FDA approval was based on NAVIGATOR trial, designed to evaluate the safety, tolerability and clinical activity of AYVAKIT® in patients with unresectable or metastatic GIST. NAVIGATOR is a multicenter, single-arm, open-label trial, which enrolled 43 patients with GIST, harboring a PDGFRA Exon 18 mutation, including 38 patients with PDGFRA D842V mutations. Patients initially enrolled in this trial received AYVAKIT® at a starting dose of 400 mg orally once daily, which was later reduced to the recommended dose of 300 mg orally once daily due to toxicity. Treatment was continued until disease progression or unacceptable toxicity. The major efficacy endpoints were Overall Response Rate (ORR) and Duration of Response.
The ORR among patients harboring a PDGFRA Exon 18 mutation was 84%, with 7% Complete Responses and 77% Partial Responses. For the subgroup of patients with PDGFRA D842V mutations, the ORR was 89%, with 8% Complete Responses and 82% Partial Responses. The median Duration of Response was not reached with a median follow up for all patients of 10.6 months. Sixty one percent (61%) of the responding patients with Exon 18 mutations had a response lasting 6 months or longer. The most common Adverse Events across all grades included nausea, vomiting, decreased appetite, diarrhea, fatigue, anemia, cognitive effects, periorbital edema, increased lacrimation and peripheral edema.
It was concluded that AYVAKIT® shows unprecedented activity in D842V and other Exon 18 mutant PDGFRA GISTs, and is the first precision therapy approved to treat a genomically defined population of patients with GIST. Heinrich M, Jones RL, von Mehren M, et al. Clinical activity of avapritinib in ≥4th line (4L+) and PDGFRA exon 18 gastrointestinal stromal tumors (GIST). J Clin Oncol. 2019;37 (suppl; abstr 11022). DOI: 10.1200/JCO.2019.37.15_suppl.11022.

