SUMMARY:The FDA in July 2014, granted Breakthrough Therapy Designation to Blinatumomab, a bispecific T cell engager (BiTE) antibody, for adults with Philadelphia-negative (Ph-) Relapsed/Refractory B-precursor Acute Lymphoblastic Leukemia (ALL). BiTE® technology engages the body’s immune system to detect and target malignant cells. These modified antibodies are designed to engage two different targets simultaneously, thereby placing the T cells within reach of the targeted cancer cell and facilitating apoptosis of the cancer cell. BiTE antibodies are currently being investigated to treat a wide variety of malignancies. Blinatumomab is an investigational BiTE® antibody designed to direct the patients T cells against CD19, a protein found on the surface of B-cell derived leukemias and lymphomas.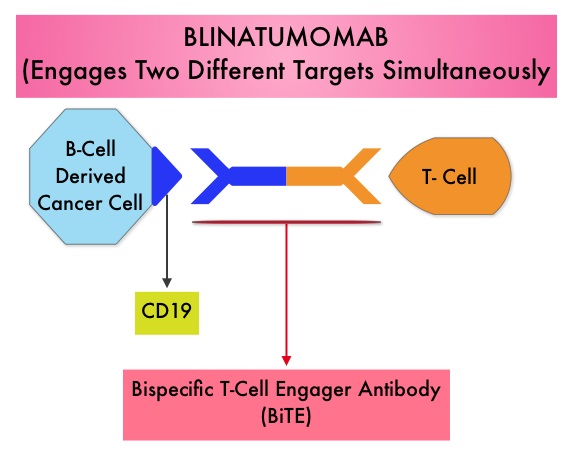 The Breakthrough Therapy Designation to Blinatumomab was based on a Phase II study in which 189 patients with Philadelphia chromosome negative ALL were enrolled. The median age was 39 years, and patients had their 1st relapse and were refractory to post hematopoietic stem cell transplantation less than 12 months before. About a third of the patients had at least 2 salvage therapies. Blinatumomab was given by continuous IV infusion, 4 weeks on and 2 weeks off for up to 5 cycles and the median number of cycles given were 2. The primary endpoint was complete remission (CR) or CR with partial hematological recovery (CRh) within the first 2 cycles of treatment. At the time of primary analysis, 43% of patients achieved a CR or CRh and 80% of responses occurred within cycle 1. Further, the Complete Remissions (CR) and CR with partial hematological recovery (CRh) were seen in all subgroups of patients, although this was more pronounced in those with less than 50% bone marrow blasts. The median Relapse Free Survival and Overall survival were 5.9 months and 6.1 months respectively. The most frequent grade 3 adverse events were febrile neutropenia, neutropenia, and anemia, occurring in 26%, 15%, and 15% of patients, respectively. The authors concluded that Blinatumomab has significant single agent antileukemia activity in a difficult-to-treat population with Relapsed and Refractory Acute Lymphoblastic Leukemia. Clinical trials will hopefully address whether Blinatumomab can serve as a bridge to transplantation, in patients with Relapsed and Refractory B-cell ALL. Topp MS, Goekbuget N, Stein AS, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 7005)</s
The Breakthrough Therapy Designation to Blinatumomab was based on a Phase II study in which 189 patients with Philadelphia chromosome negative ALL were enrolled. The median age was 39 years, and patients had their 1st relapse and were refractory to post hematopoietic stem cell transplantation less than 12 months before. About a third of the patients had at least 2 salvage therapies. Blinatumomab was given by continuous IV infusion, 4 weeks on and 2 weeks off for up to 5 cycles and the median number of cycles given were 2. The primary endpoint was complete remission (CR) or CR with partial hematological recovery (CRh) within the first 2 cycles of treatment. At the time of primary analysis, 43% of patients achieved a CR or CRh and 80% of responses occurred within cycle 1. Further, the Complete Remissions (CR) and CR with partial hematological recovery (CRh) were seen in all subgroups of patients, although this was more pronounced in those with less than 50% bone marrow blasts. The median Relapse Free Survival and Overall survival were 5.9 months and 6.1 months respectively. The most frequent grade 3 adverse events were febrile neutropenia, neutropenia, and anemia, occurring in 26%, 15%, and 15% of patients, respectively. The authors concluded that Blinatumomab has significant single agent antileukemia activity in a difficult-to-treat population with Relapsed and Refractory Acute Lymphoblastic Leukemia. Clinical trials will hopefully address whether Blinatumomab can serve as a bridge to transplantation, in patients with Relapsed and Refractory B-cell ALL. Topp MS, Goekbuget N, Stein AS, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 7005)</s
Category: Hem/Onc Updates
KEYTRUDA® – A promising Immunotherapy for Metastatic Melanoma
The FDA granted accelerated approval to KEYTRUDA® (Pembrolizumab), a humanized anti PD-1 antibody, for the treatment of patients with advanced Metastatic Melanoma, who have disease progression following YERVOY® (Ipilimumab) and if BRAF V600 mutation positive, a BRAF inhibitor. KEYTRUDA® produced significant and durable responses in patients with advanced Melanoma, regardless of prior therapy with YERVOY® and this benefit was accomplished with minimal toxicities. This new entry will revolutionize the treatment of advanced Melanoma.
Comparative Outcomes of Catheter-Directed Thrombolysis Plus Anticoagulation vs Anticoagulation Alone to Treat Lower-Extremity Proximal Deep Vein Thrombosis
SUMMARY: The Center for Disease Control and Prevention (CDC) estimates that approximately 1-2 per 1000 individuals develop Deep Vein Thrombosis/Pulmonary Embolism (PE) each year in the United States, resulting in 60,000 – 100,000 deaths. Even though Deep Vein Thrombosis (DVT) commonly occurs in the lower extremities, Non-Leg Deep Venous Thromboses (NLDVT) at other sites including the head and neck, trunk, and upper extremities can occur as well. Approximately one third of the patients with proximal Deep Vein Thrombosis (DVT) develop Post Thrombotic Syndrome which can be associated with pain and swelling in the extremity and if persistent, over time, can lead to skin pigmentation and ulceration, impacting the patients quality of life.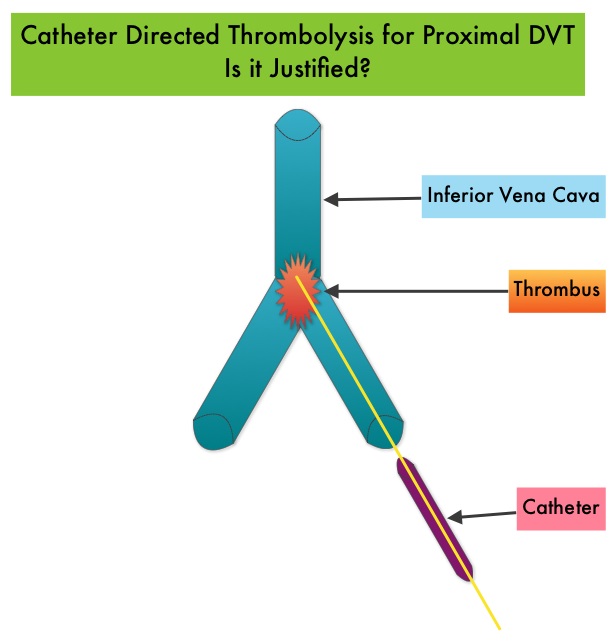 Several small clinical studies have shown a decrease in the incidence of Post Thrombotic Syndrome, with Catheter Directed Thrombolysis (CDT), although safety outcomes from this intervention remained inconclusive. This had resulted in conflicting recommendations from the American Heart Association and American College of Chest Physicians. To address this further, the authors in this study compared safety outcomes in a group of patients with proximal and caval DVT who underwent CDT plus anticoagulation with another group treated with anticoagulation alone. In this propensity-matched analysis, 90,618 patients with a discharge diagnosis of proximal DVT over a 6 year period and 3,594 well-matched patients in each study group were identified from a Nationwide Inpatient Sample (NIS) database. The primary endpoint of this study was in-hospital mortality and secondary endpoints included incidence of pulmonary embolism, blood transfusion requirements, gastrointestinal bleeding, intracranial bleeding, IVC filter placement, procedure-related hematomas, length of hospital stay and hospital charges. This analysis did not demonstrate a significant difference in the in-hospital mortality between patients who had Catheter Directed Thrombolysis (CDT) plus anticoagulation group and the anticoagulation alone group (1.2% vs 0.9%). However, the rates of blood transfusion (11.1% vs 6.5%), pulmonary embolism (17.9% vs 11.4%), intracranial hemorrhage (0.9% vs 0.3%) and IVC filter placement (34.8% vs15.6%), were significantly higher in the CDT group compared to the anticoagulation alone group. Further, patients in the CDT group had a significantly longer hospital stay (7.2 vs. 5.0 days) and incurred significantly higher hospital expenses ($85,094 vs $28,164). More patients with private insurance had Catheter Directed Thrombolysis (CDT), compared to self-pay patients or those with Medicare or Medicaid (7.3% vs 2.8%, P<0.001). The authors concluded that Catheter Directed Thrombolysis (CDT) may not improve outcomes for patients with proximal Deep Vein Thrombosis but is associated with significantly higher adverse events and therefore warrants substantial justification, if this intervention is planned. Bashir R, Zack CJ, Zhao H, et al. JAMA Intern Med. 2014;174:1494-1501
Several small clinical studies have shown a decrease in the incidence of Post Thrombotic Syndrome, with Catheter Directed Thrombolysis (CDT), although safety outcomes from this intervention remained inconclusive. This had resulted in conflicting recommendations from the American Heart Association and American College of Chest Physicians. To address this further, the authors in this study compared safety outcomes in a group of patients with proximal and caval DVT who underwent CDT plus anticoagulation with another group treated with anticoagulation alone. In this propensity-matched analysis, 90,618 patients with a discharge diagnosis of proximal DVT over a 6 year period and 3,594 well-matched patients in each study group were identified from a Nationwide Inpatient Sample (NIS) database. The primary endpoint of this study was in-hospital mortality and secondary endpoints included incidence of pulmonary embolism, blood transfusion requirements, gastrointestinal bleeding, intracranial bleeding, IVC filter placement, procedure-related hematomas, length of hospital stay and hospital charges. This analysis did not demonstrate a significant difference in the in-hospital mortality between patients who had Catheter Directed Thrombolysis (CDT) plus anticoagulation group and the anticoagulation alone group (1.2% vs 0.9%). However, the rates of blood transfusion (11.1% vs 6.5%), pulmonary embolism (17.9% vs 11.4%), intracranial hemorrhage (0.9% vs 0.3%) and IVC filter placement (34.8% vs15.6%), were significantly higher in the CDT group compared to the anticoagulation alone group. Further, patients in the CDT group had a significantly longer hospital stay (7.2 vs. 5.0 days) and incurred significantly higher hospital expenses ($85,094 vs $28,164). More patients with private insurance had Catheter Directed Thrombolysis (CDT), compared to self-pay patients or those with Medicare or Medicaid (7.3% vs 2.8%, P<0.001). The authors concluded that Catheter Directed Thrombolysis (CDT) may not improve outcomes for patients with proximal Deep Vein Thrombosis but is associated with significantly higher adverse events and therefore warrants substantial justification, if this intervention is planned. Bashir R, Zack CJ, Zhao H, et al. JAMA Intern Med. 2014;174:1494-1501
Clinical Impact of Delaying Initiation of Adjuvant Chemotherapy in Patients with Breast Cancer
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately, 233,000 new cases of invasive breast cancer will be diagnosed in 2014 and 40,000 women will die of the disease. The HER or erbB family of receptors consist of HER1, HER2, HER3 and HER4. Approximately 15%-20% of invasive breast cancers overexpress HER2/neu oncogene, which is a negative predictor of outcomes, without systemic therapy. Patients with early stage breast cancer often receive adjuvant chemotherapy and this is even more so true for HER positive and triple negative (ER, PR and HER negative) breast cancer patients, who are at an increased risk to develop recurrent disease. Even though majority of the patients start their adjuvant chemotherapy within 4-6 weeks following surgery, the impact of delay in the initiation of adjuvant therapy, on outcomes, has remained unclear. Preclinical models have suggested that there is phase of increased angiogenesis and accelerated growth of micrometastases, as well as development of drug resistant clones, following removal of the primary tumor. Previously published data from a large meta-analysis had suggested that a four week delay in the initiation of adjuvant chemotherapy resulted in a 6% increase in the risk of death and an 8% increase in the risk of relapse. Based on this background information, the authors in this study evaluated the impact of time to initiation of adjuvant chemotherapy, on survival, in patients with various stages and subtypes of early stage breast cancer. In this single institution study, 6,827 women diagnosed with stages I to III breast cancer between 1997 and 2011, were categorized into one of three groups – 30 days or less, 31 to 60 days and 61 days or more, according to the time from definitive surgery to adjuvant chemotherapy. Survival outcomes were then estimated in these three groups. The median follow up was 59.3 months and majority of the patients (84.5%) had stage I or II breast cancer and 15.5% of the patients had stage III disease. The authors noted that outcomes were inferior among patients with stage II and stage III disease when chemotherapy was initiated 61 days or more after surgery, with a 76% increase in the risk of death among patients with stage III disease. This disadvantage was however not noted in patients with stage I disease. Survival estimates based on the tumor sub types revealed that patients with triple negative breast cancer tumors and those with HER-2 positive (Human Epidermal growth factor Receptor- 2) tumors, treated 61 days or more after surgery with HERCEPTIN® (Trastuzumab) based chemotherapy, had the worse survival, compared with those who initiated adjuvant treatment within the first 30 days after surgery. Patients with hormone receptor positive tumors were however not impacted. The authors concluded that delaying the initiation of adjuvant chemotherapy in patients with high risk disease such as those with Stages II and III breast cancer and those with triple negative breast cancer and HER-2 positive tumors, can negatively impact survival outcomes. de Melo Gagliato D, Gonzalez-Angulo AM, Lei X, et al. J Clin Oncol 2014;32:735-744
Panorama 1 A randomized, double-blind, phase 3 study of panobinostat or placebo plus bortezomib and dexamethasone in relapsed or relapsed and refractory multiple myeloma
SUMMARY:Multiple Myeloma is a clonal disorder of plasma cells in the bone marrow and the American Cancer Society estimates that in the United States, 24,050 new cases will be diagnosed in 2014 and 11,090 will die of the disease. The authors in the PANORAMA I trial evaluated the outcomes in previously treated advanced multiple myeloma patients, by taking advantage of the synergy between Bortezomib (VELCADE®), a proteosome inhibitor and Panobinostat, a histone deacetylase (HDAC) inhibitor and treating these patients with a combination of these two agents. HDACs are a family of enzymes that play an important role in the regulation of gene expression.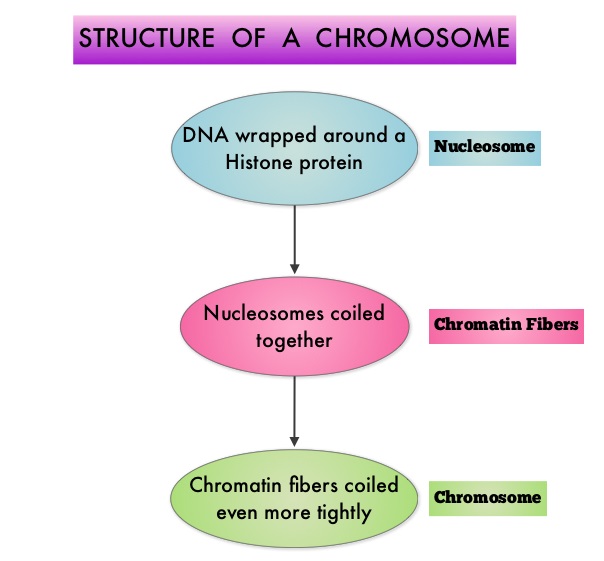 To briefly summarize the structure of a chromosome, individual loops of coiled double-helix DNA wrap around a histone protein to form a nucleosome. Nucleosomes are then coiled together to form chromatin fibers, which looks like beads on a string. The chromatin fibers are coiled even more tightly to form chromosomes. HDAC enzymes catalyze the removal of acetyl groups and regulate the level of acetylation of the histones and non-histone proteins and transcription of several genes. Hypoacetylation of histones has been associated with a condensed chromatin structure that results in the repression of gene transcription, whereas acetylated histones are associated with a more open chromatin structure and activation of gene transcription. HDACs are grouped into four major classes (Class I, II, III and IV) and regulate cell-cycle progression, cell survival, angiogenesis and immunity. The HDAC Class I enzymes are HDAC1, 2, 3 & 8 and are typically found in the nucleus where they are able to repress transcription. The HDAC Class II enzymes include HDAC4, 5, 6, 7, 9 and 10 and are able to move between the cytoplasm and nucleus and function in signal transduction. In Multiple Myeloma, the important enzyme to target is HDAC6. Panobinostat is an oral, pan-histone deacetylase inhibitor which inhibits cell cycle progression and ultimately results in apoptosis. Panobinostat inhibits the aggresome pathway of protein degradation which is upregulated when proteosome pathway is inhibited by VELCADE®.
To briefly summarize the structure of a chromosome, individual loops of coiled double-helix DNA wrap around a histone protein to form a nucleosome. Nucleosomes are then coiled together to form chromatin fibers, which looks like beads on a string. The chromatin fibers are coiled even more tightly to form chromosomes. HDAC enzymes catalyze the removal of acetyl groups and regulate the level of acetylation of the histones and non-histone proteins and transcription of several genes. Hypoacetylation of histones has been associated with a condensed chromatin structure that results in the repression of gene transcription, whereas acetylated histones are associated with a more open chromatin structure and activation of gene transcription. HDACs are grouped into four major classes (Class I, II, III and IV) and regulate cell-cycle progression, cell survival, angiogenesis and immunity. The HDAC Class I enzymes are HDAC1, 2, 3 & 8 and are typically found in the nucleus where they are able to repress transcription. The HDAC Class II enzymes include HDAC4, 5, 6, 7, 9 and 10 and are able to move between the cytoplasm and nucleus and function in signal transduction. In Multiple Myeloma, the important enzyme to target is HDAC6. Panobinostat is an oral, pan-histone deacetylase inhibitor which inhibits cell cycle progression and ultimately results in apoptosis. Panobinostat inhibits the aggresome pathway of protein degradation which is upregulated when proteosome pathway is inhibited by VELCADE®.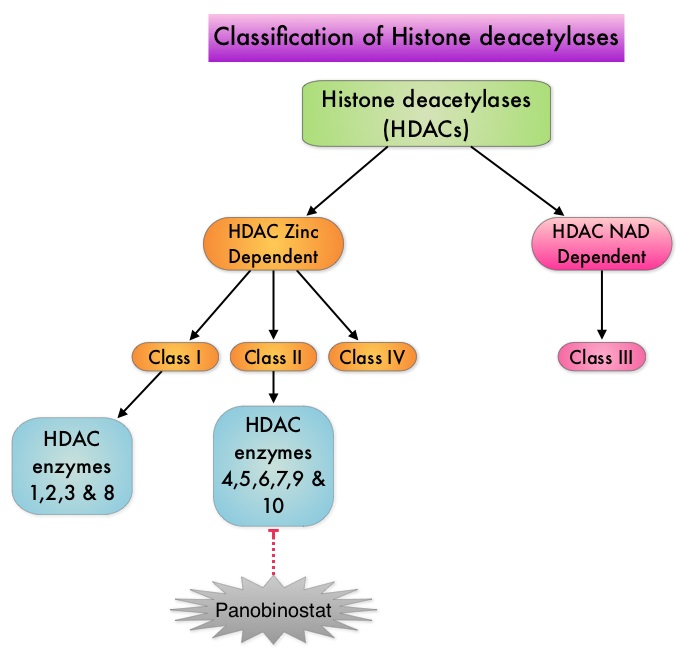 Based on preclinical data demonstrating synergy between VELCADE® and Panobinostat in Myeloma, the PANORAMA 1 trial, enrolled patients with relapsed or refractory multiple myeloma who had received one to three prior lines of therapy and were not VELCADE® refractory. In this phase III trial, patients were randomly assigned to receive either Panobinostat (N=387) or Placebo (N=381), each along with IV VELCADE® and oral Dexamethasone. For cycles 1 thru 8, patients received Panobinostat 20 mg PO or Placebo on days 1, 3, 5, 8, 10, and 12; VELCADE® 1.3 mg/m2 IV on days 1, 4, 8, and 11; and Dexamethasone 20 mg PO on days 1-2, 4-5, 8-9, and 11-12 of a 21 day cycle. Patients with clinical benefit after the first eight cycles could proceed to the second phase of treatment in which VELCADE® was administered only on D1 and D8 and Dexamethasone administered only on days 1-2 and 8-9. The median age was 63 years, 48% of patients had received at least two lines of therapy and 57% of patients had prior autologous stem cell transplantation and 43% had prior therapy with VELCADE®. The primary endpoint was Progression Free Survival (PFS) and secondary endpoints included Overall Survival (OS), Overall Response Rate (ORR), near Complete/Complete Response (nCR/CR) rate, Duration of Response (DOR), and safety. After a median follow up of 28 months, the primary end point of the study was met with a 37% decrease in the risk of disease progression in the Panobinostat group compared to the Placebo group (12 months vs 8.1 months, HR=0.63, P<0.0001). With regards to the secondary endpoints in the Panobinostat vs Placebo groups, the ORR was 60.7% vs 54.6% (P=0.87), nCR/CR rate was 27.6% vs 15.7% (P=0.00006), median duration of response was13.1months vs 10.9 months and median time to progression was 12.7 months vs 8.5 months respectively. The most common grade 3/4 adverse events in the Panobinostat vs Placebo arms included thrombocytopenia (67% vs 31%), neutropenia (35% vs 11%), and diarrhea (26% vs 8%) and these toxicities were manageable with dose reduction and supportive care. The authors concluded that a combination of Panobinostat, VELCADE® and Dexamethasone significantly improves Progression Free Survival in patients with relapsed and refractory Multiple Myeloma, with manageable toxicities. Richardson PG, Hungria VTM , Yoon S, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 8510)</s
Based on preclinical data demonstrating synergy between VELCADE® and Panobinostat in Myeloma, the PANORAMA 1 trial, enrolled patients with relapsed or refractory multiple myeloma who had received one to three prior lines of therapy and were not VELCADE® refractory. In this phase III trial, patients were randomly assigned to receive either Panobinostat (N=387) or Placebo (N=381), each along with IV VELCADE® and oral Dexamethasone. For cycles 1 thru 8, patients received Panobinostat 20 mg PO or Placebo on days 1, 3, 5, 8, 10, and 12; VELCADE® 1.3 mg/m2 IV on days 1, 4, 8, and 11; and Dexamethasone 20 mg PO on days 1-2, 4-5, 8-9, and 11-12 of a 21 day cycle. Patients with clinical benefit after the first eight cycles could proceed to the second phase of treatment in which VELCADE® was administered only on D1 and D8 and Dexamethasone administered only on days 1-2 and 8-9. The median age was 63 years, 48% of patients had received at least two lines of therapy and 57% of patients had prior autologous stem cell transplantation and 43% had prior therapy with VELCADE®. The primary endpoint was Progression Free Survival (PFS) and secondary endpoints included Overall Survival (OS), Overall Response Rate (ORR), near Complete/Complete Response (nCR/CR) rate, Duration of Response (DOR), and safety. After a median follow up of 28 months, the primary end point of the study was met with a 37% decrease in the risk of disease progression in the Panobinostat group compared to the Placebo group (12 months vs 8.1 months, HR=0.63, P<0.0001). With regards to the secondary endpoints in the Panobinostat vs Placebo groups, the ORR was 60.7% vs 54.6% (P=0.87), nCR/CR rate was 27.6% vs 15.7% (P=0.00006), median duration of response was13.1months vs 10.9 months and median time to progression was 12.7 months vs 8.5 months respectively. The most common grade 3/4 adverse events in the Panobinostat vs Placebo arms included thrombocytopenia (67% vs 31%), neutropenia (35% vs 11%), and diarrhea (26% vs 8%) and these toxicities were manageable with dose reduction and supportive care. The authors concluded that a combination of Panobinostat, VELCADE® and Dexamethasone significantly improves Progression Free Survival in patients with relapsed and refractory Multiple Myeloma, with manageable toxicities. Richardson PG, Hungria VTM , Yoon S, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 8510)</s
Electronic Cigarettes A Position Statement of the Forum of International Respiratory Societies
SUMMARY: According to the American Cancer Society, tobacco use is responsible for nearly 1 in 5 deaths in the United States and accounts for at least 30% of all cancer deaths. Smokeless tobacco products are a major source of cancer causing nitrosamines and increase the risk of developing cancer of the oropharynx, esophagus, and pancreas. Cigarette smoke contains more than 7,000 chemicals, many of which are toxic and some linked to cancer. E-cigarettes were first developed in China and were introduced to the U.S. market in 2007. When a smoker inhales through the mouth piece of an E-cigarette, the air flow triggers a sensor that switches on a small lithium battery powered heater, which in turn vaporizes liquid nicotine along with PolyEthylene Glycol (PEG) present in a small cartridge. The PEG vapor looks like smoke. The potent liquid form of nicotine extracted from tobacco is tinctured with fragrant flavors such as chocolate, cherry and bubble gum, coloring substances, as well other chemicals and these e-liquids are powerful neurotoxins. With the rapid growth of the E-cigarette industry and the evidence of potential dangers and risk to public health, particularly children, experts from the world's leading lung organizations were compelled to release a position statement on electronic cigarettes, specifically focusing on their potential adverse effects on human health and calling on government organizations to ban or restrict the use of E-cigarettes, until their impact on health is better understood. With epidemiological data demonstrating that nicotine use is a gateway to the use of cocaine and marijuana and subsequent lifelong addiction, the Forum of International Respiratory Societies (FIRS), an organization composed of the world's leading international respiratory societies including American Thoracic Society (ATS) and the American College of Chest Physicians (ACCP), made the following recommendations. The position statement of the Forum of International Respiratory Societies (FIRS) on electronic nicotine delivery devices includes the following:
When a smoker inhales through the mouth piece of an E-cigarette, the air flow triggers a sensor that switches on a small lithium battery powered heater, which in turn vaporizes liquid nicotine along with PolyEthylene Glycol (PEG) present in a small cartridge. The PEG vapor looks like smoke. The potent liquid form of nicotine extracted from tobacco is tinctured with fragrant flavors such as chocolate, cherry and bubble gum, coloring substances, as well other chemicals and these e-liquids are powerful neurotoxins. With the rapid growth of the E-cigarette industry and the evidence of potential dangers and risk to public health, particularly children, experts from the world's leading lung organizations were compelled to release a position statement on electronic cigarettes, specifically focusing on their potential adverse effects on human health and calling on government organizations to ban or restrict the use of E-cigarettes, until their impact on health is better understood. With epidemiological data demonstrating that nicotine use is a gateway to the use of cocaine and marijuana and subsequent lifelong addiction, the Forum of International Respiratory Societies (FIRS), an organization composed of the world's leading international respiratory societies including American Thoracic Society (ATS) and the American College of Chest Physicians (ACCP), made the following recommendations. The position statement of the Forum of International Respiratory Societies (FIRS) on electronic nicotine delivery devices includes the following:
• The safety of electronic cigarettes has not been adequately demonstrated.
• The addictive power of nicotine and its untoward effects should not be underestimated.
• The potential benefits of electronic nicotine delivery devices, including harm reduction and as an aid to smoking cessation, have not been well studied.
• Potential benefits to an individual smoker should be weighed against harm to the population of increased social acceptability of smoking and use of nicotine.
• Health and safety claims regarding electronic nicotine delivery devices should be subject to evidentiary review.
• Adverse health effects for third parties exposed to the emissions of electronic cigarettes cannot be excluded.
• Electronic nicotine delivery devices should be restricted or banned, at least until more information about their safety is available.
• If electronic nicotine delivery devices are permitted, they should be regulated as medicines and subject to the same evidentiary review of other medicines.
• If electronic nicotine delivery devices are not regulated as medicines, they should be regulated as tobacco products.
• Research, supported by sources other than the tobacco or electronic cigarette industry, should be carried out to determine the impact of electronic nicotine delivery devices on health in a wide variety of settings.
• The use and population effects of electronic nicotine delivery devices should be monitored.
• All information derived from this research should be conveyed to the public in a clear manner.
Schraufnagel DE, Blasi F, Drummond MB, et al. on behalf of the Forum of International Respiratory Societies. Am J Respir Crit Care Med. First published online 09 Jul 2014 as DOI: 10.1164/rccm.201407-1198PP
Randomized, Controlled, Double-Blind, Cross-Over Trial Assessing Treatment Preference for Pazopanib Versus Sunitinib in Patients With Metastatic Renal Cell Carcinoma PISCES Study
SUMMARY: Kidney cancer is among the 10 most common cancers in both men and women and the American Cancer Society estimates that approximately 64,000 new cases of kidney cancer will be diagnosed for 2014 in the United States and about 13,860 individuals will die from this disease. Renal cell carcinoma is a highly vascular tumor. Vascular Endothelial Growth Factor (VEGF), PDGF (Platelet Derived Growth Factor) and FGF (Fibroblast Growth Factor) are the major growth factors involved in angiogenesis, with VEGF playing the most important role, regulating vascular permeability. These ligands (VEGF, PDGF and FGF) bind to their respective receptors which exhibit tyrosine kinase activity and are able to activate the signaling pathways down stream. The study of Von Hippel-Lindau (VHL) syndrome and the cloning of VHL gene (a tumor suppressor gene) led to the understanding of the molecular basis and biology of the most common type of sporadic Renal Cell Carcinoma (Clear Cell Carcinoma). All patients with VHL syndrome have a germ line inactivating mutation of one VHL allele and inactivation of the second allele is the triggering event for the formation of Clear Cell Carcinomas of the kidney, as well as other tumors including endocrine Pancreatic tumors, Papillary cystadenomas of the pancreas, adnexal organs, epididymis, endolymphatic system of the inner ear, Hemangioblastoma of the CNS and retina and Pheochromocytomas. It appears that close to 60% of the patients with non-hereditary (sporadic) clear cell carcinoma of the kidney have loss of function of the VHL gene.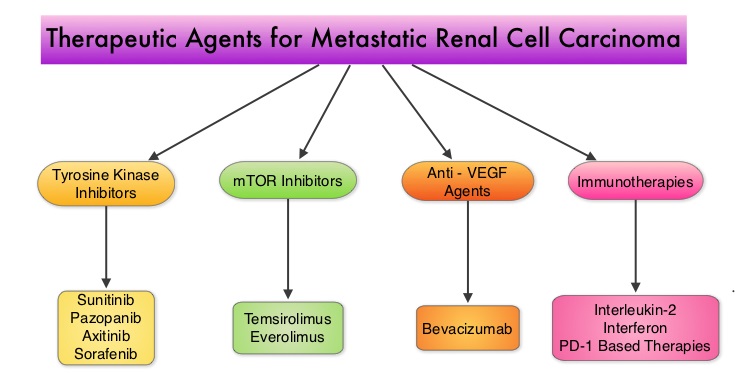 The understanding of the biology of clear cell carcinoma of the kidney has led to the development of multitargeted Tyrosine Kinase Inhibitors, which are small molecules that simultaneously target the tumor cell wall, vascular endothelial cell wall as well as the pericyte/fibroblast/vascular/ smooth vessel cell wall. The end result is decreased angiogenesis, cell proliferation and cell survival of both endothelial cells as well as tumor cells. There are four classes of agents presently available for the treatment of metastatic Renal Cell carcinoma. They include Immunotherapies, Anti-VEGF agents, Tyrosine Kinase Inhibitors (TKI’s) and mTOR (Mammalian Target of Rapamycin) inhibitors. The choice of First Line therapies for metastatic Renal Cell Carcinoma is based on MSKCC (Memorial Sloan Kettering Cancer Center) risk classification and the agents include, Temsirolimus (TORISEL®) for poor risk patients and TKI’s as well as Immunotherapy with or without anti-VEGF agents for good and intermediate risk patients.
The understanding of the biology of clear cell carcinoma of the kidney has led to the development of multitargeted Tyrosine Kinase Inhibitors, which are small molecules that simultaneously target the tumor cell wall, vascular endothelial cell wall as well as the pericyte/fibroblast/vascular/ smooth vessel cell wall. The end result is decreased angiogenesis, cell proliferation and cell survival of both endothelial cells as well as tumor cells. There are four classes of agents presently available for the treatment of metastatic Renal Cell carcinoma. They include Immunotherapies, Anti-VEGF agents, Tyrosine Kinase Inhibitors (TKI’s) and mTOR (Mammalian Target of Rapamycin) inhibitors. The choice of First Line therapies for metastatic Renal Cell Carcinoma is based on MSKCC (Memorial Sloan Kettering Cancer Center) risk classification and the agents include, Temsirolimus (TORISEL®) for poor risk patients and TKI’s as well as Immunotherapy with or without anti-VEGF agents for good and intermediate risk patients. 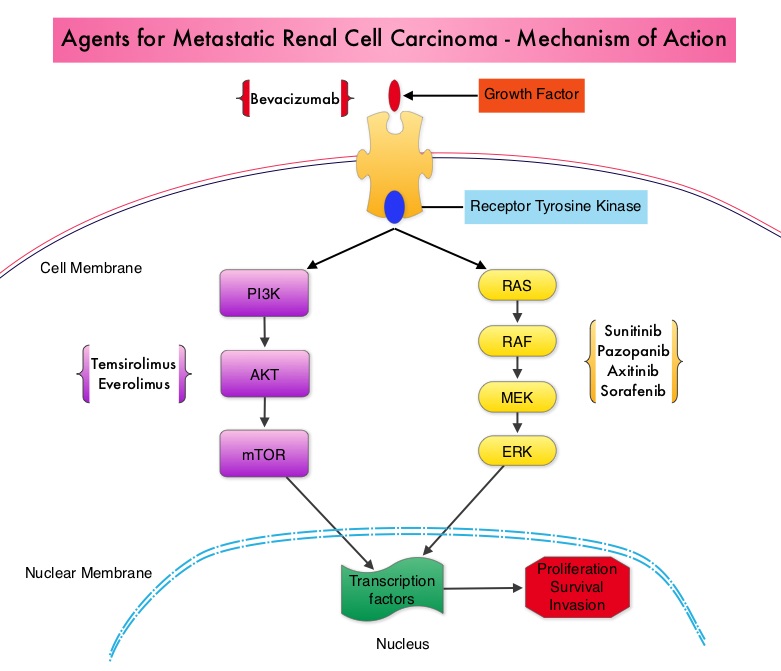 The TKI’s of choice however for good to intermediate risk mRCC patients with clear cell histology are, either SUTENT® (Sunitinib) or VOTRIENT® (Pazopanib). Even though both agents have similar efficacy, it remained unclear if one agent was better tolerated than the other. To address this, the authors evaluated patient preference for VOTRIENT® or SUTENT® in a double blind, phase III cross over study. The primary end point of this study was patient preference for a specific treatment, as assessed by a questionnaire at the end of the two treatment periods. Other endpoints included reasons for preference, physician preference, safety, and HRQoL (Health Related Quality of Life). Randomly assigned patients with metastatic Renal Cell Carcinoma (mRCC) with clear cell histology (N=168), received VOTRIENT® 800 mg per day for 10 weeks followed by a 2 week washout period and then SUTENT® 50 mg per day (4 weeks on, 2 weeks off, 4 weeks on) for 10 weeks (N=86), or the reverse sequence (N=82). One hundred and fourteen (N=114) patients met the prespecified modified intent-to-treat criteria for the primary analysis and the criteria included exposure to both of the treatments, no disease progression before cross over and completion of the preference questionnaire. When outcomes were evaluated, 70% of the patients preferred VOTRIENT®, 22% preferred SUTENT® and 8% had no preference (P<0.001).
The TKI’s of choice however for good to intermediate risk mRCC patients with clear cell histology are, either SUTENT® (Sunitinib) or VOTRIENT® (Pazopanib). Even though both agents have similar efficacy, it remained unclear if one agent was better tolerated than the other. To address this, the authors evaluated patient preference for VOTRIENT® or SUTENT® in a double blind, phase III cross over study. The primary end point of this study was patient preference for a specific treatment, as assessed by a questionnaire at the end of the two treatment periods. Other endpoints included reasons for preference, physician preference, safety, and HRQoL (Health Related Quality of Life). Randomly assigned patients with metastatic Renal Cell Carcinoma (mRCC) with clear cell histology (N=168), received VOTRIENT® 800 mg per day for 10 weeks followed by a 2 week washout period and then SUTENT® 50 mg per day (4 weeks on, 2 weeks off, 4 weeks on) for 10 weeks (N=86), or the reverse sequence (N=82). One hundred and fourteen (N=114) patients met the prespecified modified intent-to-treat criteria for the primary analysis and the criteria included exposure to both of the treatments, no disease progression before cross over and completion of the preference questionnaire. When outcomes were evaluated, 70% of the patients preferred VOTRIENT®, 22% preferred SUTENT® and 8% had no preference (P<0.001). 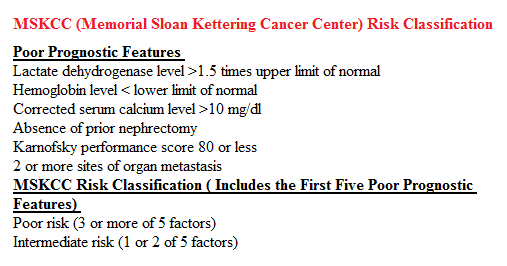 The main reasons for preferring VOTRIENT® were less fatigue and better overall quality of life whereas those who preferred SUTENT® cited less diarrhea as the main reason. Physician preference was also taken into consideration in this study, as physicians are able to better assess efficacy and asymptomatic toxicities that are clinically relevant. VOTRIENT® was preferred by 61% of the physicians, 22% preferred SUTENT® and 17% had no preference. VOTRIENT® was also superior to SUTENT® with regards to HRQoL measures that evaluated fatigue, hand/foot soreness and mouth/throat soreness. The authors concluded that this innovative cross-over trial demonstrated that significantly more patients preferred VOTRIENT® over SUTENT®, based on lower rates of adverse events and better Health-Related Quality of Life. Escudier B, Porta C, Bono P, et al. J Clin Oncol 2014;32:1412-1418
The main reasons for preferring VOTRIENT® were less fatigue and better overall quality of life whereas those who preferred SUTENT® cited less diarrhea as the main reason. Physician preference was also taken into consideration in this study, as physicians are able to better assess efficacy and asymptomatic toxicities that are clinically relevant. VOTRIENT® was preferred by 61% of the physicians, 22% preferred SUTENT® and 17% had no preference. VOTRIENT® was also superior to SUTENT® with regards to HRQoL measures that evaluated fatigue, hand/foot soreness and mouth/throat soreness. The authors concluded that this innovative cross-over trial demonstrated that significantly more patients preferred VOTRIENT® over SUTENT®, based on lower rates of adverse events and better Health-Related Quality of Life. Escudier B, Porta C, Bono P, et al. J Clin Oncol 2014;32:1412-1418
ZYDELIG® – A New Treatment Option for Non-Hodgkin Lymphoma
The FDA granted ZYDELIG®, accelerated approval in relapsed Follicular B-cell Non-Hodgkin Lymphoma and Small Lymphocytic Lymphoma. The approval was based on ZYDELIG®’s significant single agent activity with an acceptable safety profile, in heavily pretreated patients with indolent Non Hodgkin Lymphomas. ZYDELIG® is a highly selective, small molecule, oral inhibitor of the enzyme Phosphoinositide 3-Kinase delta (PI3K delta) and blocks the delta isoform of PI3K enzyme and its signaling pathway, thus promoting apoptosis. More information is available at www.oncoprescribe.com
Phase III trial (Prevention of Early Menopause Study [POEMS]-SWOG S0230) of LHRH analog during chemotherapy (CT) to reduce ovarian failure in early-stage, hormone receptor-negative breast cancer An international Intergroup trial of SWOG, IBCSG, ECOG, and CALGB (Alliance)
SUMMARY:Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately, 233,000 new cases of invasive breast cancer will be diagnosed in 2014 and 40,000 women will die of the disease. Approximately 75% of patients with breast cancer are hormone receptor positive (Estrogen Receptor/Progesterone Receptor positive) and this is a predictor of response to endocrine therapy. In premenopausal woman, the ovary is the main source of estrogen production, whereas in postmenopausal women, the primary source of estrogen is the Aromatase enzyme mediated conversion of androstenedione and testosterone to estrone and estradiol in extragonadal/peripheral tissues. Premature Ovarian Failure (POF) is a common unintended consequence of chemotherapy in premenopausal women. Besides of loss of fertility, which can influence treatment decisions in young women, ovarian failure can lead to menopausal symptoms, sexual dysfunction and loss of bone density. 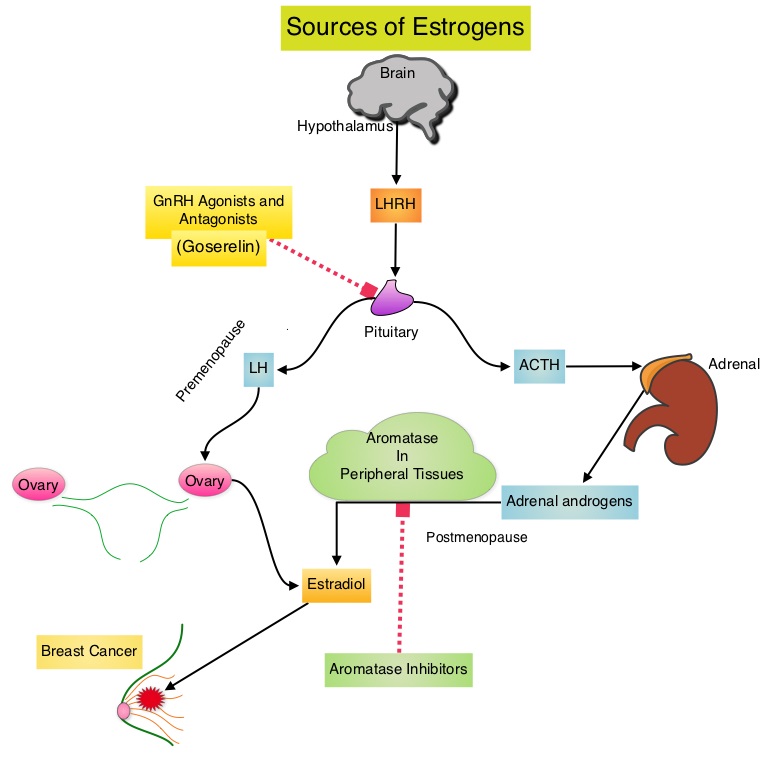 POEMS (Prevention of Early Menopause Study) is a randomized phase III trial designed to evaluate whether the addition of LHRH (Luteinizing Hormone-Releasing Hormone) analog Goserelin (ZOLADEX®), which suppresses the production of estrogens, to Cyclophosphamide based chemotherapy, would reduce POF in breast cancer patients, when compared to chemotherapy alone. Premenopausal patients less than 50 years of age, with hormone negative (ER/PR negative ), Stage I-IIIA breast cancer, scheduled to receive chemotherapy, were randomly assigned to receive standard Cyclophosphamide based chemotherapy with or without monthly ZOLADEX® . Patients in the ZOLADEX® group received 3.6 mg SQ starting 1 week prior to the first dose of chemotherapy. The primary endpoint was ovarian failure at two years (defined as amenorrhea for the prior 6 months AND post-menopausal FSH level). Other endpoints included pregnancy and survival rates. Of the 218 evaluable patients, 135 premenopausal women were evaluable for the primary end point. POF rates were 22% in the chemotherapy alone group and 8% in the ZOLADEX® group (P=0.03). When the definition of POF was more liberal to include EITHER amenorrhea or elevated FSH but not both, POF rates were 45% in the chemotherapy alone group and 20% in the ZOLADEX® group (P=0.006). Among the 218 evaluable patients, more women in the ZOLADEX® group achieved at least one pregnancy (21%) compared to 11% in the chemotherapy alone group (P=0.03). Secondary outcomes also favored the ZOLADEX® group with a Disease free Survival (DFS) rate of 78% in the chemotherapy alone group compared with 89% in the ZOLADEX® group (P=0.04) and Overall Survival (OS) rate of 82% in the chemotherapy alone group compared with 92% in the ZOLADEX® group (P=0.05). The authors concluded that the addition of ZOLADEX® to chemotherapy improved fertility prospects with a lower incidence of Premature Ovarian Failure and more pregnancies. Further, the improved Disease Free Survival and Overall Survival is an important additional perk and prevention of POF with ZOLADEX® may be a consideration not only in premenopausal patients with hormone receptor positive breast cancer but also in other malignancies such as lymphomas, when treated with similar chemotherapeutic agents. Moore HC, Unger JM, Phillips K, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr LBA505)</s
POEMS (Prevention of Early Menopause Study) is a randomized phase III trial designed to evaluate whether the addition of LHRH (Luteinizing Hormone-Releasing Hormone) analog Goserelin (ZOLADEX®), which suppresses the production of estrogens, to Cyclophosphamide based chemotherapy, would reduce POF in breast cancer patients, when compared to chemotherapy alone. Premenopausal patients less than 50 years of age, with hormone negative (ER/PR negative ), Stage I-IIIA breast cancer, scheduled to receive chemotherapy, were randomly assigned to receive standard Cyclophosphamide based chemotherapy with or without monthly ZOLADEX® . Patients in the ZOLADEX® group received 3.6 mg SQ starting 1 week prior to the first dose of chemotherapy. The primary endpoint was ovarian failure at two years (defined as amenorrhea for the prior 6 months AND post-menopausal FSH level). Other endpoints included pregnancy and survival rates. Of the 218 evaluable patients, 135 premenopausal women were evaluable for the primary end point. POF rates were 22% in the chemotherapy alone group and 8% in the ZOLADEX® group (P=0.03). When the definition of POF was more liberal to include EITHER amenorrhea or elevated FSH but not both, POF rates were 45% in the chemotherapy alone group and 20% in the ZOLADEX® group (P=0.006). Among the 218 evaluable patients, more women in the ZOLADEX® group achieved at least one pregnancy (21%) compared to 11% in the chemotherapy alone group (P=0.03). Secondary outcomes also favored the ZOLADEX® group with a Disease free Survival (DFS) rate of 78% in the chemotherapy alone group compared with 89% in the ZOLADEX® group (P=0.04) and Overall Survival (OS) rate of 82% in the chemotherapy alone group compared with 92% in the ZOLADEX® group (P=0.05). The authors concluded that the addition of ZOLADEX® to chemotherapy improved fertility prospects with a lower incidence of Premature Ovarian Failure and more pregnancies. Further, the improved Disease Free Survival and Overall Survival is an important additional perk and prevention of POF with ZOLADEX® may be a consideration not only in premenopausal patients with hormone receptor positive breast cancer but also in other malignancies such as lymphomas, when treated with similar chemotherapeutic agents. Moore HC, Unger JM, Phillips K, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr LBA505)</s
PI3Kδ Inhibition by Idelalisib in Patients with Relapsed Indolent Lymphoma
SUMMARY: Non-Hodgkin Lymphoma (NHL) is one of the most common cancers in the United States and the American Cancer Society estimates that in 2014, about 70,800 people will be diagnosed with NHL in the US and close to 19,000 people will die of the disease. PI3K delta signaling is hyperactive in B-cell malignancies and is important for the activation, proliferation, homing of malignant B cells in the lymphoid tissues and their survival. PI3K (PhosphatidylInositol 3-Kinase) is a lipid kinase and has four distinct isoforms – alpha, beta, gamma and delta. The alpha and beta isoforms are expressed in a wide variety of tissues whereas the gamma and delta isoforms are only expressed in hematopoietic cells. PI3K delta (the delta isoform of PI3K enzyme) is predominantly expressed in leukocytes and plays an important role in the normal B lymphocyte development as well as signal transduction from B cell receptor as well as receptors for various cytokines and chemokines.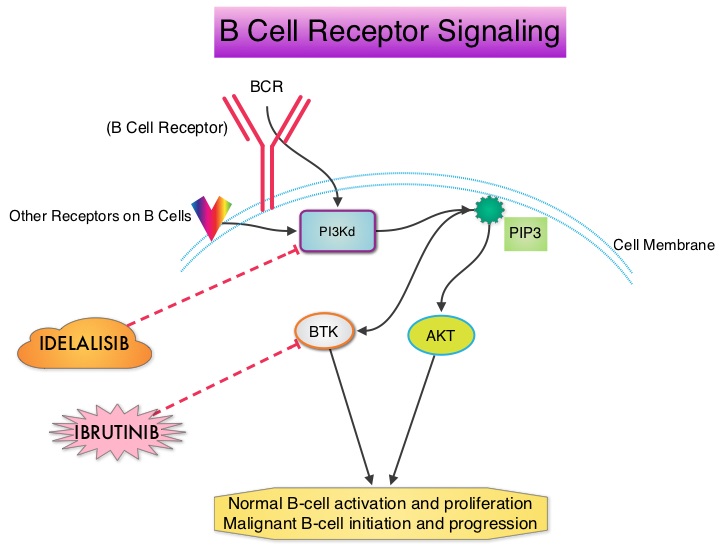 PI3K delta is activated by BCR signaling resulting in the production of a second messenger, Phosphatidylinositol 3,4,5-triphosphate (PIP3) which in turn activates Bruton’s Tyrosine Kinase (BTK) and AKT, a prosurvival kinase. Idelalisib (ZYDELIG®) is a highly selective, small molecule, oral inhibitor of the enzyme Phosphoinositide 3-Kinase delta (PI3K delta) and blocks the delta isoform of PI3K enzyme and its signaling pathway, thus promoting apoptosis. The FDA granted ZYDELIG® accelerated approval in relapsed Follicular B-cell Non-Hodgkin Lymphoma and Small Lymphocytic Lymphoma based on the results of a single-arm, open-label, phase II trial. Patients with indolent Non-Hodgkin Lymphomas (N=125), who were refractory to Rituximab (RITUXAN®) and an alkylating agent or had relapsed within 6 months after receipt of these therapies, received ZYDELIG®, 150 mg PO BID. Treatment was continued until disease progression or unacceptable toxicities developed. The median age was 64 years and enrolled patients had received a median of four prior therapies. The indolent Non-Hodgkin Lymphoma subtypes included Follicular lymphoma (N=72), Small Lymphocytic Lymphoma (N=28), Marginal Zone Lymphoma (N=15), and Lymphoplasmacytic Lymphoma with or without Waldenström's Macroglobulinemia (N=10). The primary end point of this study was Overall Response Rate and secondary end points included the Duration of Response, Progression Free Survival, and Safety. The median follow up was 9.7 months. The Overall Response Rate was 57% with 50% partial responses and 6% complete responses. There was no difference in the Reponse Rates across the various subtypes of Indolent Non-Hodgkin Lymphomas. The median time to response was 1.9 months and the median duration of response was 12.5 months. The median Progression Free Survival was 11 months and the Overall Survival at one year was estimated to be 80%. The most common grade 3 or higher adverse events were diarrhea (13%), neutropenia (27%) and elevations in SGOT and SGPT levels (13%). These toxicities were manageable with dose modifications and dose interruptions. The authors concluded that ZYDELIG® has significant single agent activity, with an acceptable safety profile, in heavily pretreated patients with indolent Non Hodgkin Lymphomas. Gopal AK, Kahl BS, de Vos S, et al. N Engl J Med 2014; 370:1008-1018
PI3K delta is activated by BCR signaling resulting in the production of a second messenger, Phosphatidylinositol 3,4,5-triphosphate (PIP3) which in turn activates Bruton’s Tyrosine Kinase (BTK) and AKT, a prosurvival kinase. Idelalisib (ZYDELIG®) is a highly selective, small molecule, oral inhibitor of the enzyme Phosphoinositide 3-Kinase delta (PI3K delta) and blocks the delta isoform of PI3K enzyme and its signaling pathway, thus promoting apoptosis. The FDA granted ZYDELIG® accelerated approval in relapsed Follicular B-cell Non-Hodgkin Lymphoma and Small Lymphocytic Lymphoma based on the results of a single-arm, open-label, phase II trial. Patients with indolent Non-Hodgkin Lymphomas (N=125), who were refractory to Rituximab (RITUXAN®) and an alkylating agent or had relapsed within 6 months after receipt of these therapies, received ZYDELIG®, 150 mg PO BID. Treatment was continued until disease progression or unacceptable toxicities developed. The median age was 64 years and enrolled patients had received a median of four prior therapies. The indolent Non-Hodgkin Lymphoma subtypes included Follicular lymphoma (N=72), Small Lymphocytic Lymphoma (N=28), Marginal Zone Lymphoma (N=15), and Lymphoplasmacytic Lymphoma with or without Waldenström's Macroglobulinemia (N=10). The primary end point of this study was Overall Response Rate and secondary end points included the Duration of Response, Progression Free Survival, and Safety. The median follow up was 9.7 months. The Overall Response Rate was 57% with 50% partial responses and 6% complete responses. There was no difference in the Reponse Rates across the various subtypes of Indolent Non-Hodgkin Lymphomas. The median time to response was 1.9 months and the median duration of response was 12.5 months. The median Progression Free Survival was 11 months and the Overall Survival at one year was estimated to be 80%. The most common grade 3 or higher adverse events were diarrhea (13%), neutropenia (27%) and elevations in SGOT and SGPT levels (13%). These toxicities were manageable with dose modifications and dose interruptions. The authors concluded that ZYDELIG® has significant single agent activity, with an acceptable safety profile, in heavily pretreated patients with indolent Non Hodgkin Lymphomas. Gopal AK, Kahl BS, de Vos S, et al. N Engl J Med 2014; 370:1008-1018
