SUMMARY: Non-Hodgkin Lymphoma (NHL) is one of the most common cancers in the United States and the American Cancer Society estimates that in 2014, about 70,800 people will be diagnosed with NHL in the US and close to 19,000 people will die of the disease. RITUXAN® (Rituximab) is a first generation type I, chimeric anti-CD20 targeted monoclonal antibody that destroys malignant human B cells primarily by complement-dependent cytotoxicity (CDC) and Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC).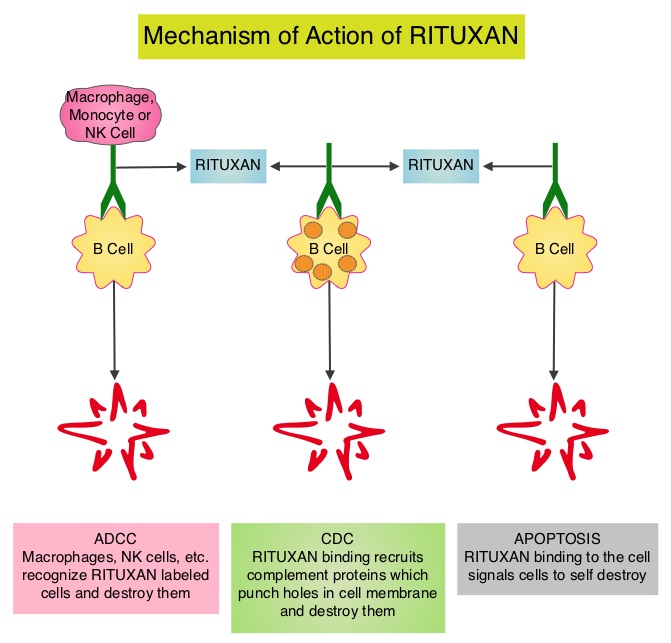 Since its approval in 1997, immunochemotherapy regimens incorporating RITUXAN® has had a major impact in treatment outcomes for patients with Follicular Lymphomas both in first line as well as relapsed settings. Two years of RITUXAN® maintenance therapy after induction immunochemotherapy as first-line treatment for high tumor burden Follicular Lymphoma, significantly improved Progression Free Survival, as was shown in the PRIMA study. Similarly, maintenance RITUXAN® has been shown to improve Progression Free Survival when compared with observation, in patients with low tumor burden Follicular Lymphoma. Whether maintenance RITUXAN® provides superior long term disease control compared with retreatment with RITUXAN® when disease progression is noted, has remained unclear. RESORT [Rituximab Extended Schedule or Re-Treatment Trial] is a randomized trial designed to determine whether maintenance treatment with RITUXAN® provided superior disease control compared with retreatment with RITUXAN® at disease progression, in patients with previously untreated low tumor burden Follicular Lymphoma. Low tumor burden was defined as no mass more than 7 cm, fewer than three masses more than 3 cm, no B symptoms, spleen size less than 16 cm by CT scan, no evidence of organ compromise, circulating lymphocytes less 5,000/μL, and no evidence of cytopenias defined as platelets less than 100,000/μL, hemoglobin less than 10 g/dL, or absolute neutrophil count less than 1,500/μL. Of the 408 patients with Follicular Lymphoma included in this study, 289 patients responded to induction treatment with 4 weekly doses of RITUXAN® given at 375mg/m2. These patients were then randomly assigned to maintenance RITUXAN® (N= 146) or retreatment with RITUXAN® (N=143) at each disease progression, until treatment failure. Maintenance RITUXAN® treatment consisted of a single dose of RITUXAN® given every 3 months until treatment failure. The primary end point of this study was time to treatment failure. Secondary end points included time to first cytotoxic therapy, toxicity, and health-related quality of life (HRQOL). With a median follow-up of 4.5 years, there was no difference in the median time to treatment failure amongst the maintenance RITUXAN® and retreatment RITUXAN® groups (4.3 years vs 3.9 years, P=0.54). The median number of RITUXAN® doses was 18 for those receiving maintenance RITUXAN® compared to 4 for those receiving retreatment RITUXAN®. Grade 3 or 4 toxicities were uncommon in both treatment groups and there was no difference in health-related quality of life. The authors concluded that in low tumor burden Follicular Lymphoma, a retreatment strategy at disease progression utilizes fewer doses of RITUXAN® with outcomes equivalent to that achieved with maintenance RITUXAN®. Kahl BS, Hong F, Williams ME, et al. J Clin Oncol 2014;32:3096-3102
Since its approval in 1997, immunochemotherapy regimens incorporating RITUXAN® has had a major impact in treatment outcomes for patients with Follicular Lymphomas both in first line as well as relapsed settings. Two years of RITUXAN® maintenance therapy after induction immunochemotherapy as first-line treatment for high tumor burden Follicular Lymphoma, significantly improved Progression Free Survival, as was shown in the PRIMA study. Similarly, maintenance RITUXAN® has been shown to improve Progression Free Survival when compared with observation, in patients with low tumor burden Follicular Lymphoma. Whether maintenance RITUXAN® provides superior long term disease control compared with retreatment with RITUXAN® when disease progression is noted, has remained unclear. RESORT [Rituximab Extended Schedule or Re-Treatment Trial] is a randomized trial designed to determine whether maintenance treatment with RITUXAN® provided superior disease control compared with retreatment with RITUXAN® at disease progression, in patients with previously untreated low tumor burden Follicular Lymphoma. Low tumor burden was defined as no mass more than 7 cm, fewer than three masses more than 3 cm, no B symptoms, spleen size less than 16 cm by CT scan, no evidence of organ compromise, circulating lymphocytes less 5,000/μL, and no evidence of cytopenias defined as platelets less than 100,000/μL, hemoglobin less than 10 g/dL, or absolute neutrophil count less than 1,500/μL. Of the 408 patients with Follicular Lymphoma included in this study, 289 patients responded to induction treatment with 4 weekly doses of RITUXAN® given at 375mg/m2. These patients were then randomly assigned to maintenance RITUXAN® (N= 146) or retreatment with RITUXAN® (N=143) at each disease progression, until treatment failure. Maintenance RITUXAN® treatment consisted of a single dose of RITUXAN® given every 3 months until treatment failure. The primary end point of this study was time to treatment failure. Secondary end points included time to first cytotoxic therapy, toxicity, and health-related quality of life (HRQOL). With a median follow-up of 4.5 years, there was no difference in the median time to treatment failure amongst the maintenance RITUXAN® and retreatment RITUXAN® groups (4.3 years vs 3.9 years, P=0.54). The median number of RITUXAN® doses was 18 for those receiving maintenance RITUXAN® compared to 4 for those receiving retreatment RITUXAN®. Grade 3 or 4 toxicities were uncommon in both treatment groups and there was no difference in health-related quality of life. The authors concluded that in low tumor burden Follicular Lymphoma, a retreatment strategy at disease progression utilizes fewer doses of RITUXAN® with outcomes equivalent to that achieved with maintenance RITUXAN®. Kahl BS, Hong F, Williams ME, et al. J Clin Oncol 2014;32:3096-3102
Category: Hem/Onc Updates
Everolimus (EVE) for the treatment of advanced pancreatic neuroendocrine tumors (pNET) Final overall survival (OS) results of a randomized, double-blind, placebo (PBO)-controlled, multicenter Phase III trial (RADIANT-3) SUMMARY Pancreatic NeuroEndocrine Tumors (PNETs) are uncommon, slow growing neoplasms and their incidence has been on the rise due to heightened awareness of the disease, better diagnostic techniques and increased rate of incidental diagnosis during workup for other conditions They account for approximately 25% of all neuroendocrine tumors Pancreatic NETs may be functional or nonfunctional Functional tumors secrete hormones, such as gastrin, insulin, and glucagon which may be associated with symptoms and signs whereas nonfunctional tumors, which account for 90% of PNETs, do not produce extra amounts of hormones Majority of the functional tumors are benign whereas more than 50% of the nonfunctional tumors are malignant and are often advanced at the time of diagnosis as they do not produce hormones and associated symptoms Patients with metastatic PNETs have a poor prognosis with a median survival of 1-3 years, similar to that of metastatic breast cancer and metastatic colon cancer Predictors of worst survival include advanced stage, higher grade and age Treatment options have included surgery if feasible, Somatostatin analogues, combination chemotherapy with limited success, hepatic artery embolization and availability of two new agents, Everolimus (AFINITOR®), a mTOR (mammalian Target Of Rapamycin) inhibitor and Sunitinib (SUTENT®), a multitargeted Tyrosine Kinase Inhibitor RADIANT-3 is a randomized, double-blind, phase III study in which 410 patients with advanced, low grade or intermediate grade Pancreatic NeuroEndocrine Tumors with progression within the previous 12 months, were randomized to receive AFINITOR® at a dose of 10 mg PO daily (207 patients) or placebo (203 patients), in addition to best supportive care The primary endpoint was Progression Free Survival, and the secondary endpoints included Overall Survival and the safety and tolerability of AFINITOR® Upon progression, patients in the placebo group were allowed to cross over and receive open-label AFINITOR® Further, when all patients were unblinded at the end of the predetermined cutoff date, those in the placebo group were offered open-label AFINITOR® and those in the AFINITOR® arm continued to receive open-label AFINITOR® The authors had reported the results from the primary analysis of this study in 2011 when the primary end point of Progression Free Survival (PFS) was met, with a PFS of 11 months in the AFINITOR® group and 46 months in the placebo group (P < 0001) The authors now report the mature Overall Survival results The median Overall Survival was 44 months in the AFINITOR® group compared with 377 months in the placebo arm The 63 month survival difference in favor of AFINITOR® was not statistically significant (P= 030) The authors attributed the lack of statistically significant survival benefit, to crossover of 85% of patients from placebo to AFINITOR® group, which may have confounded the ability to detect a statistically significant survival advantage with AFINITOR® The most common adverse events associated with AFINITOR® were stomatitis, rash, diarrhea and fatigue The authors concluded that median Overall Survival of 44 months with AFINITOR® for advanced progressive Pancreatic NeuroEndocrine Tumors is unprecedented, confirming that the mTOR pathway plays an important role in the molecular pathogenesis of Pancreatic NeuroEndocrine Tumors Yao J, Pavel M, Lombard-Bohas C, et al ESMO 2014 Congress, Abstract#11320
They account for approximately 25% of all neuroendocrine tumors Pancreatic NETs may be functional or nonfunctional Functional tumors secrete hormones, such as gastrin, insulin, and glucagon which may be associated with symptoms and signs whereas nonfunctional tumors, which account for 90% of PNETs, do not produce extra amounts of hormones Majority of the functional tumors are benign whereas more than 50% of the nonfunctional tumors are malignant and are often advanced at the time of diagnosis as they do not produce hormones and associated symptoms Patients with metastatic PNETs have a poor prognosis with a median survival of 1-3 years, similar to that of metastatic breast cancer and metastatic colon cancer Predictors of worst survival include advanced stage, higher grade and age Treatment options have included surgery if feasible, Somatostatin analogues, combination chemotherapy with limited success, hepatic artery embolization and availability of two new agents, Everolimus (AFINITOR®), a mTOR (mammalian Target Of Rapamycin) inhibitor and Sunitinib (SUTENT®), a multitargeted Tyrosine Kinase Inhibitor RADIANT-3 is a randomized, double-blind, phase III study in which 410 patients with advanced, low grade or intermediate grade Pancreatic NeuroEndocrine Tumors with progression within the previous 12 months, were randomized to receive AFINITOR® at a dose of 10 mg PO daily (207 patients) or placebo (203 patients), in addition to best supportive care The primary endpoint was Progression Free Survival, and the secondary endpoints included Overall Survival and the safety and tolerability of AFINITOR® Upon progression, patients in the placebo group were allowed to cross over and receive open-label AFINITOR® Further, when all patients were unblinded at the end of the predetermined cutoff date, those in the placebo group were offered open-label AFINITOR® and those in the AFINITOR® arm continued to receive open-label AFINITOR® The authors had reported the results from the primary analysis of this study in 2011 when the primary end point of Progression Free Survival (PFS) was met, with a PFS of 11 months in the AFINITOR® group and 46 months in the placebo group (P < 0001) The authors now report the mature Overall Survival results The median Overall Survival was 44 months in the AFINITOR® group compared with 377 months in the placebo arm The 63 month survival difference in favor of AFINITOR® was not statistically significant (P= 030) The authors attributed the lack of statistically significant survival benefit, to crossover of 85% of patients from placebo to AFINITOR® group, which may have confounded the ability to detect a statistically significant survival advantage with AFINITOR® The most common adverse events associated with AFINITOR® were stomatitis, rash, diarrhea and fatigue The authors concluded that median Overall Survival of 44 months with AFINITOR® for advanced progressive Pancreatic NeuroEndocrine Tumors is unprecedented, confirming that the mTOR pathway plays an important role in the molecular pathogenesis of Pancreatic NeuroEndocrine Tumors Yao J, Pavel M, Lombard-Bohas C, et al ESMO 2014 Congress, Abstract#11320
SUMMARY: Pancreatic NeuroEndocrine Tumors (PNETs) are uncommon, slow growing neoplasms and their incidence has been on the rise due to heightened awareness of the disease, better diagnostic techniques and increased rate of incidental diagnosis during workup for other conditions.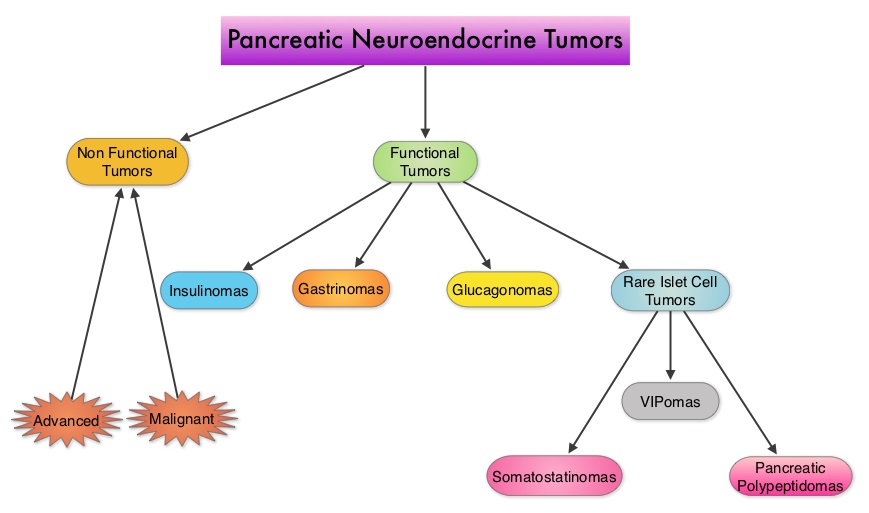 They account for approximately 25% of all neuroendocrine tumors. Pancreatic NETs may be functional or nonfunctional. Functional tumors secrete hormones, such as gastrin, insulin, and glucagon which may be associated with symptoms and signs whereas nonfunctional tumors, which account for 90% of PNETs, do not produce extra amounts of hormones. Majority of the functional tumors are benign whereas more than 50% of the nonfunctional tumors are malignant and are often advanced at the time of diagnosis as they do not produce hormones and associated symptoms. Patients with metastatic PNETs have a poor prognosis with a median survival of 1-3 years, similar to that of metastatic breast cancer and metastatic colon cancer. Predictors of worst survival include advanced stage, higher grade and age. Treatment options have included surgery if feasible, Somatostatin analogues, combination chemotherapy with limited success, hepatic artery embolization and availability of two new agents, Everolimus (AFINITOR®), a mTOR (mammalian Target Of Rapamycin) inhibitor and Sunitinib (SUTENT®), a multitargeted Tyrosine Kinase Inhibitor. RADIANT-3 is a randomized, double-blind, phase III study in which 410 patients with advanced, low grade or intermediate grade Pancreatic NeuroEndocrine Tumors with progression within the previous 12 months, were randomized to receive AFINITOR® at a dose of 10 mg PO daily (207 patients) or placebo (203 patients), in addition to best supportive care. The primary endpoint was Progression Free Survival, and the secondary endpoints included Overall Survival and the safety and tolerability of AFINITOR®. Upon progression, patients in the placebo group were allowed to cross over and receive open-label AFINITOR®. Further, when all patients were unblinded at the end of the predetermined cutoff date, those in the placebo group were offered open-label AFINITOR® and those in the AFINITOR® arm continued to receive open-label AFINITOR®. The authors had reported the results from the primary analysis of this study in 2011 when the primary end point of Progression Free Survival (PFS) was met, with a PFS of 11 months in the AFINITOR® group and 4.6 months in the placebo group (P < 0.001). The authors now report the mature Overall Survival results. The median Overall Survival was 44 months in the AFINITOR® group compared with 37.7 months in the placebo arm. The 6.3 month survival difference in favor of AFINITOR® was not statistically significant (P= 0.30). The authors attributed the lack of statistically significant survival benefit, to crossover of 85% of patients from placebo to AFINITOR® group, which may have confounded the ability to detect a statistically significant survival advantage with AFINITOR®. The most common adverse events associated with AFINITOR® were stomatitis, rash, diarrhea and fatigue. The authors concluded that median Overall Survival of 44 months with AFINITOR® for advanced progressive Pancreatic NeuroEndocrine Tumors is unprecedented, confirming that the mTOR pathway plays an important role in the molecular pathogenesis of Pancreatic NeuroEndocrine Tumors. Yao J, Pavel M, Lombard-Bohas C, et al. ESMO 2014 Congress, Abstract#11320
They account for approximately 25% of all neuroendocrine tumors. Pancreatic NETs may be functional or nonfunctional. Functional tumors secrete hormones, such as gastrin, insulin, and glucagon which may be associated with symptoms and signs whereas nonfunctional tumors, which account for 90% of PNETs, do not produce extra amounts of hormones. Majority of the functional tumors are benign whereas more than 50% of the nonfunctional tumors are malignant and are often advanced at the time of diagnosis as they do not produce hormones and associated symptoms. Patients with metastatic PNETs have a poor prognosis with a median survival of 1-3 years, similar to that of metastatic breast cancer and metastatic colon cancer. Predictors of worst survival include advanced stage, higher grade and age. Treatment options have included surgery if feasible, Somatostatin analogues, combination chemotherapy with limited success, hepatic artery embolization and availability of two new agents, Everolimus (AFINITOR®), a mTOR (mammalian Target Of Rapamycin) inhibitor and Sunitinib (SUTENT®), a multitargeted Tyrosine Kinase Inhibitor. RADIANT-3 is a randomized, double-blind, phase III study in which 410 patients with advanced, low grade or intermediate grade Pancreatic NeuroEndocrine Tumors with progression within the previous 12 months, were randomized to receive AFINITOR® at a dose of 10 mg PO daily (207 patients) or placebo (203 patients), in addition to best supportive care. The primary endpoint was Progression Free Survival, and the secondary endpoints included Overall Survival and the safety and tolerability of AFINITOR®. Upon progression, patients in the placebo group were allowed to cross over and receive open-label AFINITOR®. Further, when all patients were unblinded at the end of the predetermined cutoff date, those in the placebo group were offered open-label AFINITOR® and those in the AFINITOR® arm continued to receive open-label AFINITOR®. The authors had reported the results from the primary analysis of this study in 2011 when the primary end point of Progression Free Survival (PFS) was met, with a PFS of 11 months in the AFINITOR® group and 4.6 months in the placebo group (P < 0.001). The authors now report the mature Overall Survival results. The median Overall Survival was 44 months in the AFINITOR® group compared with 37.7 months in the placebo arm. The 6.3 month survival difference in favor of AFINITOR® was not statistically significant (P= 0.30). The authors attributed the lack of statistically significant survival benefit, to crossover of 85% of patients from placebo to AFINITOR® group, which may have confounded the ability to detect a statistically significant survival advantage with AFINITOR®. The most common adverse events associated with AFINITOR® were stomatitis, rash, diarrhea and fatigue. The authors concluded that median Overall Survival of 44 months with AFINITOR® for advanced progressive Pancreatic NeuroEndocrine Tumors is unprecedented, confirming that the mTOR pathway plays an important role in the molecular pathogenesis of Pancreatic NeuroEndocrine Tumors. Yao J, Pavel M, Lombard-Bohas C, et al. ESMO 2014 Congress, Abstract#11320
Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors vs Conventional Chemotherapy in Non–Small Cell Lung Cancer Harboring Wild-Type Epidermal Growth Factor Receptor – A Meta-analysis
SUMMARY: Lung cancer is the second most common cancer in both men and women and accounts for about 13% of all new cancers and 27% of all cancer deaths. It is the leading cause of cancer death among both men and women. The American Cancer Society estimates that over 224,000 new cases of lung cancer will be diagnosed in the United States in 2014 and over 159,000 will die of the disease. With changes in the cigarette composition and decline in tobacco consumption over the past several decades, adenocarcinoma now is the most frequent histologic subtype of lung cancer.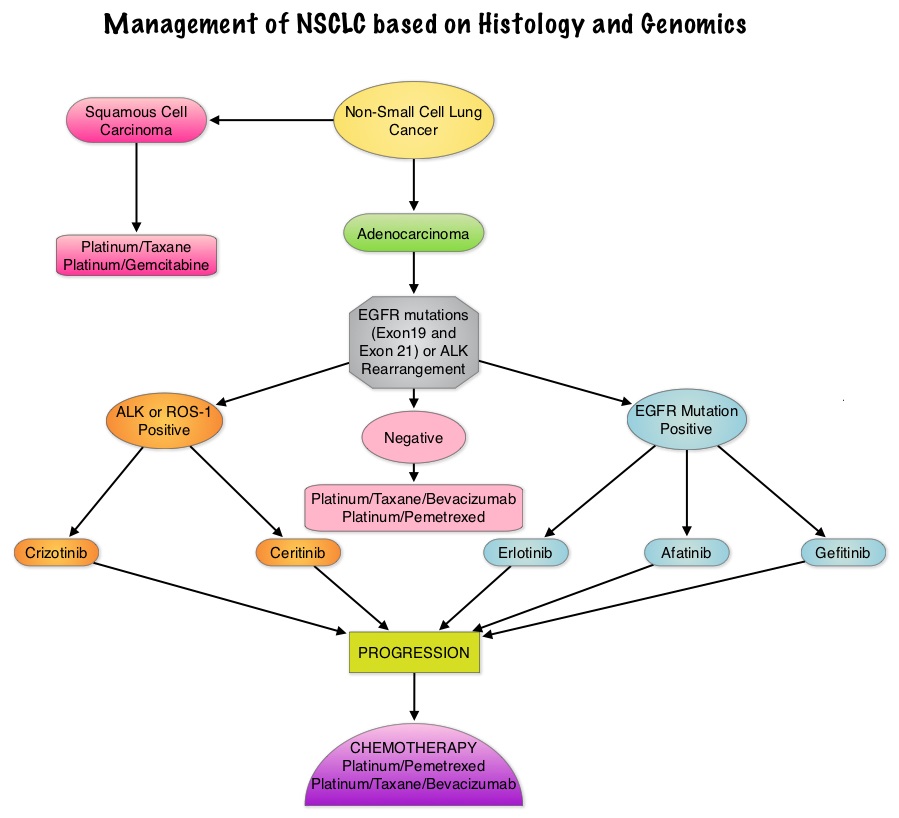 In 2004, the discovery of Epidermal Growth Factor Receptor (EGFR) mutations in some advanced Non Small Cell Lung Cancer (NSCLC) cases with adenocarcinoma histology and the favorable responses with EGFR Tyrosine Kinase Inhibitors (TKIs) such as TARCEVA® (Erlotinib) and IRESSA® (Gefitinib), changed the treatment paradigm in favor of targeted therapy, for this patient subset. It is estimated that approximately 10% of Western patient population and 50% of Asian patients with NSCLC, harbor EGFR activating mutations. EGFR Tyrosine Kinase Inhibitors have been shown to be superior to conventional chemotherapy in this patient group with improved Progression Free Survival (PFS) and Objective Response Rates. Patients with NSCLC should therefore be tested for the most common sensitizing mutations such as deletions in exon 19 and L858R point mutations in exon 21, as these patients clearly benefit from first line EGFR TKIs. EGFR expression by IHC (ImmunoHistoChemical) staining, EGFR gene copy by FISH (Fluorescence In Situ Hybridization) and blood based proteonomic testing by VERISTRAT® is currently not recommended for the selection of first line EGFR TKIs. There is presently no evidence indicating superiority of TKIs when compared with conventional chemotherapy for the second or third line treatment of EGFR Wild Type NSCLC. Nonetheless, TKIs are often recommended due to their acceptable toxicities. To address this treatment dilemma, the authors performed a systematic review and meta-analysis of randomized controlled trials, comparing first-generation EGFR TKIs (TARCEVA® and IRESSA®) treatment with conventional chemotherapy, in patients with advanced NSCLC, harboring Wild Type EGFR. This pooled analysis included 1605 patients from 11 clinical trials, with EGFR Wild Type NSCLC. The primary outcome measured was Progression Free Survival (PFS) and secondary outcomes were Objective Response Rate and Overall Survival. It was noted in this analysis that conventional chemotherapy was associated with longer PFS, compared with EGFR TKIs, among patients harboring Wild Type EGFR tumors. The authors noted that there was significant PFS benefit with chemotherapy, in trials using more sensitive EGFR mutation analysis platforms, than direct Sanger sequencing, and this may be the result of identifying the “true” Wild Type EGFR tumors. The objective response rate was higher at 16.8% with chemotherapy versus 7.2% for TKIs. There was however no statistically significant difference in the overall survival between the chemotherapy and TKI groups. When subgroups of patients in these trials were analyzed, outcomes were similar regardless of line of treatment, dominant ethinicity or EGFR mutation analysis method. The lack of improvement in Overall Survival in the chemotherapy group has been attributed to the large cross over rates in the trials that were analyzed. The authors concluded that conventional chemotherapy is associated with superior Progression Free Survival and Objective Response Rates, in patients with advanced NSCLC, harboring Wild Type EGFR tumors, compared with EGFR TKIs and the present guidelines recommending EGFR TKIs in this patient group has to be reevaluated. Lee J, Hahn S, Kim D, et al. JAMA 2014;311:1430-1437
In 2004, the discovery of Epidermal Growth Factor Receptor (EGFR) mutations in some advanced Non Small Cell Lung Cancer (NSCLC) cases with adenocarcinoma histology and the favorable responses with EGFR Tyrosine Kinase Inhibitors (TKIs) such as TARCEVA® (Erlotinib) and IRESSA® (Gefitinib), changed the treatment paradigm in favor of targeted therapy, for this patient subset. It is estimated that approximately 10% of Western patient population and 50% of Asian patients with NSCLC, harbor EGFR activating mutations. EGFR Tyrosine Kinase Inhibitors have been shown to be superior to conventional chemotherapy in this patient group with improved Progression Free Survival (PFS) and Objective Response Rates. Patients with NSCLC should therefore be tested for the most common sensitizing mutations such as deletions in exon 19 and L858R point mutations in exon 21, as these patients clearly benefit from first line EGFR TKIs. EGFR expression by IHC (ImmunoHistoChemical) staining, EGFR gene copy by FISH (Fluorescence In Situ Hybridization) and blood based proteonomic testing by VERISTRAT® is currently not recommended for the selection of first line EGFR TKIs. There is presently no evidence indicating superiority of TKIs when compared with conventional chemotherapy for the second or third line treatment of EGFR Wild Type NSCLC. Nonetheless, TKIs are often recommended due to their acceptable toxicities. To address this treatment dilemma, the authors performed a systematic review and meta-analysis of randomized controlled trials, comparing first-generation EGFR TKIs (TARCEVA® and IRESSA®) treatment with conventional chemotherapy, in patients with advanced NSCLC, harboring Wild Type EGFR. This pooled analysis included 1605 patients from 11 clinical trials, with EGFR Wild Type NSCLC. The primary outcome measured was Progression Free Survival (PFS) and secondary outcomes were Objective Response Rate and Overall Survival. It was noted in this analysis that conventional chemotherapy was associated with longer PFS, compared with EGFR TKIs, among patients harboring Wild Type EGFR tumors. The authors noted that there was significant PFS benefit with chemotherapy, in trials using more sensitive EGFR mutation analysis platforms, than direct Sanger sequencing, and this may be the result of identifying the “true” Wild Type EGFR tumors. The objective response rate was higher at 16.8% with chemotherapy versus 7.2% for TKIs. There was however no statistically significant difference in the overall survival between the chemotherapy and TKI groups. When subgroups of patients in these trials were analyzed, outcomes were similar regardless of line of treatment, dominant ethinicity or EGFR mutation analysis method. The lack of improvement in Overall Survival in the chemotherapy group has been attributed to the large cross over rates in the trials that were analyzed. The authors concluded that conventional chemotherapy is associated with superior Progression Free Survival and Objective Response Rates, in patients with advanced NSCLC, harboring Wild Type EGFR tumors, compared with EGFR TKIs and the present guidelines recommending EGFR TKIs in this patient group has to be reevaluated. Lee J, Hahn S, Kim D, et al. JAMA 2014;311:1430-1437
Final overall survival analysis from the Cleopatra study of first-line pertuzumab, trastuzumab and docetaxel in patients with HER2-positive metastatic breast cancer
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately, 233,000 new cases of invasive breast cancer will be diagnosed in 2014 and 40,000 women will die of the disease. The HER or erbB family of receptors consist of HER1, HER2, HER3 and HER4. Approximately 15%-20% of invasive breast cancers overexpress HER2/neu oncogene, which is a negative predictor of outcomes without systemic therapy. HERCEPTIN® (Trastuzumab) is a humanized monoclonal antibody targeting HER2. Trastuzumab binds to subdomain IV of the HER2 extracellular domain and blocks the downstream cell signaling pathways (PI3K-AKT pathway) and induces Antibody Dependent Cellular Cytotoxicity (ADCC). HERCEPTIN® in combination with chemotherapy has been proven to significantly improve Progression Free Survival and Overall Survival in patients with advanced breast cancer. Despite this benefit, majority of these patients develop progressive disease within 18 months. The tumors in these patients continue to express HER2 although the lower sensitivity to HER2 targeted agents has been attributed to HER2 independent escape mechanisms. Treatment strategies for this patient population have included switching chemotherapy in subsequent lines of treatment and continuing HERCEPTIN®, combining another HER2 targeted agent, Lapatinib (TYKERB®) with Capecitabine (XELODA®) and dual HER2 inhibition with a combination of HERCEPTIN® and TYKERB®.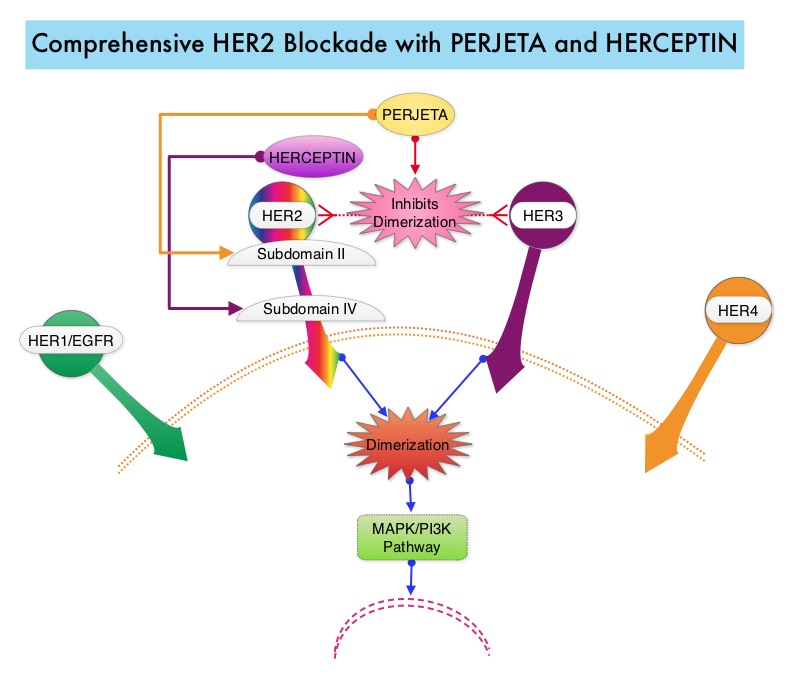 PERJETA® (Pertuzumab) is a recombinant humanized monoclonal antibody that binds to the HER2 at a different epitope of the HER2 extracellular domain (subdomain II) compared to HERCEPTIN® and prevents the dimerization of HER2 with HER3 receptor. PERJETA® stimulates antibody-dependent, cell-mediated cytotoxicity similar to HERCEPTIN®. By combining HERCEPTIN® and PERJETA®, a more comprehensive blockade of HER2 signaling can be accomplished, as these two agents bind to different HER2 epitopes and may complement each other and improve efficacy, as was demonstrated in early phase trials. The CLEOPATRA trial is a phase III study in which 808 treatment naive HER positive metastatic breast cancer patients, were randomly assigned to receive either HERCEPTIN® plus Docetaxel or this two drug combination given along with PERJETA®. PERJETA® was given as an 840 mg loading dose followed by a 420 mg maintenance dose, HERCEPTIN® was given as an 8 mg/kg loading dose followed by a 6 mg/kg maintenance dose and Docetaxel was given at 75 mg/m2 for at least 6 cycles. Treatment was administered every 3 weeks and continued until disease progression. The primary end point of this study was Progression Free Survival and secondary end points included Overall Survival, objective response rate and safety. A previous analysis performed in May 2012 showed that the addition of PERJETA® to the combination of HERCEPTIN® and Docetaxel significantly prolonged Progression Free Survival compared to HERCEPTIN® plus Docetaxel alone (18.5 months vs 12.4 months) but the median overall survival had not been reached then. In this final Overall Survival analysis, at a median follow up of 50 months, median Overall Survival was 56.5 months with the PERJETA® combination compared to 40.8 months in the non-PERJETA® group (hazard ratio [HR] = 0.68; P=0.0002). This meant that adding PERJETA® to HERCEPTIN® and Docetaxel increased the median Overall Survival by 15.7 months. The increase in Progression Free Survival by 6.3 months with the PERJETA® combination, was again maintained (HR = 0.68, P < 0.0001) at the time of the final analysis. The incidence of symptomatic left ventricular dysfunction as well as declines in left ventricular ejection fraction, were rare and similar between the two treatment groups. Based on the CLEOPATRA study, women with HER positive metastatic breast cancer, should be considered candidates, for treatment with a combination of PERJETA®, HERCEPTIN® and Docetaxel. Swain S, Kim S, Cortes J, et al. Presented at: the 2014 Congress of the European Society of Medical Oncology; September 26-30, 2014; Madrid, Spain. Abstract 350O
PERJETA® (Pertuzumab) is a recombinant humanized monoclonal antibody that binds to the HER2 at a different epitope of the HER2 extracellular domain (subdomain II) compared to HERCEPTIN® and prevents the dimerization of HER2 with HER3 receptor. PERJETA® stimulates antibody-dependent, cell-mediated cytotoxicity similar to HERCEPTIN®. By combining HERCEPTIN® and PERJETA®, a more comprehensive blockade of HER2 signaling can be accomplished, as these two agents bind to different HER2 epitopes and may complement each other and improve efficacy, as was demonstrated in early phase trials. The CLEOPATRA trial is a phase III study in which 808 treatment naive HER positive metastatic breast cancer patients, were randomly assigned to receive either HERCEPTIN® plus Docetaxel or this two drug combination given along with PERJETA®. PERJETA® was given as an 840 mg loading dose followed by a 420 mg maintenance dose, HERCEPTIN® was given as an 8 mg/kg loading dose followed by a 6 mg/kg maintenance dose and Docetaxel was given at 75 mg/m2 for at least 6 cycles. Treatment was administered every 3 weeks and continued until disease progression. The primary end point of this study was Progression Free Survival and secondary end points included Overall Survival, objective response rate and safety. A previous analysis performed in May 2012 showed that the addition of PERJETA® to the combination of HERCEPTIN® and Docetaxel significantly prolonged Progression Free Survival compared to HERCEPTIN® plus Docetaxel alone (18.5 months vs 12.4 months) but the median overall survival had not been reached then. In this final Overall Survival analysis, at a median follow up of 50 months, median Overall Survival was 56.5 months with the PERJETA® combination compared to 40.8 months in the non-PERJETA® group (hazard ratio [HR] = 0.68; P=0.0002). This meant that adding PERJETA® to HERCEPTIN® and Docetaxel increased the median Overall Survival by 15.7 months. The increase in Progression Free Survival by 6.3 months with the PERJETA® combination, was again maintained (HR = 0.68, P < 0.0001) at the time of the final analysis. The incidence of symptomatic left ventricular dysfunction as well as declines in left ventricular ejection fraction, were rare and similar between the two treatment groups. Based on the CLEOPATRA study, women with HER positive metastatic breast cancer, should be considered candidates, for treatment with a combination of PERJETA®, HERCEPTIN® and Docetaxel. Swain S, Kim S, Cortes J, et al. Presented at: the 2014 Congress of the European Society of Medical Oncology; September 26-30, 2014; Madrid, Spain. Abstract 350O
The association of indoor tanning and melanoma in adults systematic review and meta-analysis
SUMMARY: It is estimated that in the US, approximately 74,000 new cases of melanoma will be diagnosed and close to 10,000 individuals will die of the disease in 2015. The incidence of melanoma has been on the rise for the past three decades. A major risk factor for most skin cancers is exposure to UltraViolet (UV) radiation, which damages the DNA of skin cells. The main source of UV rays is sunlight, tanning lamps and tanning beds. The 3 main types of UV rays include UVA rays, UVB rays that mainly cause sunburns and UVC rays that do not penetrate through our atmosphere and are not in sunlight. Most indoor tanning beds give off large amounts of UVA rays, which have been found to increase skin cancer risk. It appears that there are no safe UV rays. The International Agency for Research on Cancer has classified Indoor tanning as a Class I carcinogen based on its significant association with malignant melanoma. Indoor tanning with resulting exposure to ultraviolet radiation is a potentially modifiable behavior and several studies to date have shown a relationship between indoor tanning and skin cancer. With this background, the authors reviewed the literature on indoor tanning and gathered data from 31 studies published in peer-reviewed journals that provided risk estimates. These studies included 14,956 cases with malignant melanoma and 233,106 controls. The main focus was to determine the risk of melanoma based on the frequency of use and exposure to the newer indoor tanning beds. They noted that among North Americans, there was a 34% increased risk of developing melanoma in individuals attending more than 10 tanning sessions. Further, those who started indoor tanning before age 25 years, had a 35% risk of developing melanoma and those who ever used indoor tanning were at a 23% increased risk of developing melanoma. It is hypothesized that the newer tanning bed bulb technology, which emits larger doses of long wave UVA rays, has resulted in a 22% increase in the risk of melanoma in individuals who ever used indoor tanning after the year 2000, compared to only 12% in the same population group before the year 2000. The authors concluded that the newer tanning technology is not safer than older techniques and patients should be educated and informed that using tanning beds can be associated with a subsequent diagnosis of malignant melanoma and therefore, cessation of indoor tanning should be strongly encouraged. Colantonio S, Bracken MB, Beecker J. J Am Acad Dermatol. 2014;70:847-857
The International Agency for Research on Cancer has classified Indoor tanning as a Class I carcinogen based on its significant association with malignant melanoma. Indoor tanning with resulting exposure to ultraviolet radiation is a potentially modifiable behavior and several studies to date have shown a relationship between indoor tanning and skin cancer. With this background, the authors reviewed the literature on indoor tanning and gathered data from 31 studies published in peer-reviewed journals that provided risk estimates. These studies included 14,956 cases with malignant melanoma and 233,106 controls. The main focus was to determine the risk of melanoma based on the frequency of use and exposure to the newer indoor tanning beds. They noted that among North Americans, there was a 34% increased risk of developing melanoma in individuals attending more than 10 tanning sessions. Further, those who started indoor tanning before age 25 years, had a 35% risk of developing melanoma and those who ever used indoor tanning were at a 23% increased risk of developing melanoma. It is hypothesized that the newer tanning bed bulb technology, which emits larger doses of long wave UVA rays, has resulted in a 22% increase in the risk of melanoma in individuals who ever used indoor tanning after the year 2000, compared to only 12% in the same population group before the year 2000. The authors concluded that the newer tanning technology is not safer than older techniques and patients should be educated and informed that using tanning beds can be associated with a subsequent diagnosis of malignant melanoma and therefore, cessation of indoor tanning should be strongly encouraged. Colantonio S, Bracken MB, Beecker J. J Am Acad Dermatol. 2014;70:847-857
A phase 2, randomized trial of GVAX pancreas and CRS-207 immunotherapy versus GVAX alone in patients with metastatic pancreatic adenocarcinoma Updated results
SUMMARY: The American Cancer Society estimates that in 2014, over 46,000 people will be diagnosed with Pancreatic Cancer in the United States and close to 40,000 people will die of the disease. Some important risk factors for Pancreatic Cancer include increasing age, obesity, smoking history, genetic predisposition, exposure to certain dyes and chemicals, heavy alcohol use and pancreatitis. The best chance for long term survival is complete surgical resection, although this may not be feasible in a majority of the patients, as they present with advanced disease at the time of diagnosis. Based on the National Cancer Data Base, the 5 year observed survival rate for patients diagnosed with exocrine cancer of the pancreas is 14% for those with Stage IA disease and 1% for those with Stage IV disease. The FDA recently granted Breakthrough Therapy Designation status for the combination treatment that consists of two vaccines, GVAX and CRS-207, for patients with advanced Pancreatic Carcinoma. This designation was based on the following study. A phase II clinical trial was conducted in which the authors took a novel approach and tested a combination of two vaccines in patients with metastatic pancreatic adenocarcinoma.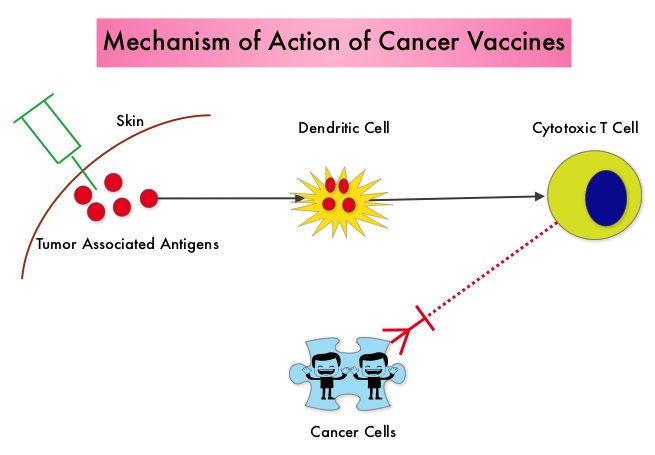 Traditional vaccination against specific bacterial and viral infections involves the injection of the specific weakened bacteria/virus or a structural component of the bacteria or virus. The body then mounts an immune response and is ready to respond to an infection associated with that specific bacteria or virus. Use of vaccines in cancer treatment is based on the same principle. The two vaccines studied were GVAX and CRS-207. GVAX is an allogeneic whole cell vaccine developed from pancreatic cancer cell lines. These cancer cells are irradiated, to prevent them from dividing and are genetically modified to secrete GM-CSF (Granulocyte Macrophage Colony Stimulating Factor). GM-CSF is important for the growth and activation of dendritic cells also known as Antigen Presenting Cells. This vaccine when injected attracts the dendritic cells to the vaccine injection site and the dendritic cells in turn, pick up the antigens from the vaccine and present them to the patient’s immune system. The immune system then mounts a response by activating tumor specific T-cells. This vaccine therefore theoretically boosts the body’s immune system to fight the patient’s tumor, without causing collateral damage. The second vaccine CRS-207 is live-attenuated (weakened) Listeria monocytogenes bacterium which expresses mesothelin and stimulates innate and adaptive immunity. It is genetically engineered to elicit an immune response against the tumor-associated antigen mesothelin, which has been shown to be expressed at higher levels on pancreatic cancer cells than on normal cells. Previous studies have demonstrated that survival can be improved by induction of mesothelin specific T-cell responses. In this study, 90 patients with metastatic pancreatic adenocarcinoma were randomly assigned in a 2:1 ratio to receive two doses of GVAX followed by four doses of CRS-207 or six doses of GVAX alone. Treatment was given every 3 weeks and low-dose CYTOXAN® (Cyclophosphamide) was given IV, the day before GVAX in both groups, to inhibit regulatory (suppressive) T-cells. More than 80% of the patients had at least one prior treatment for metastatic disease and 50% had two or more prior treatments. The primary endpoint was overall survival. Secondary endpoints included safety, clinical and immune responses. At a planned interim analysis, the median overall survival was 6.1 months with the combination of two vaccines vs 3.9 months with GVAX alone (HR=0.54, P=0.011), a 46% reduction in risk of death with the combination immunotherapy. The median overall survival in patients who received three total doses which included at least two doses of GVAX and at least one dose of CRS-207 was 9.7 months compared to 4.6 months for GVAX alone (HR=0.44, P=0.0074), a 56% reduction in the risk of death. In the subgroup of patients who had two or more prior chemotherapy regimens, combination immunotherapy given as third line therapy or greater resulted in a median overall survival of 5.1 months vs 3.7 months with GVAX alone (HR=0.34, P=0.001), a 66% reduction in risk of death. Stabilization of tumor marker CA19-9, was seen in 32% of patients receiving combination immunotherapy vs 13% in those who received GVAX alone (P=0.06). The one year survival probability doubled with the dual vaccine with an estimated one year survival of 24% for the combination immunotherapy group and 12% for the GVAX alone group. Toxicities included local reactions after GVAX and transient fevers, chills, and lymphopenia after CRS-207 administration. The authors concluded that immunotherapy with a combination of two vaccines improved overall survival in patients with metastatic pancreatic carcinoma, who had failed prior therapies. Le DT, Wang-Gillam A, Picozzi V, et al. J Clin Oncol 32, 2014 (suppl 3; abstr 177)
Traditional vaccination against specific bacterial and viral infections involves the injection of the specific weakened bacteria/virus or a structural component of the bacteria or virus. The body then mounts an immune response and is ready to respond to an infection associated with that specific bacteria or virus. Use of vaccines in cancer treatment is based on the same principle. The two vaccines studied were GVAX and CRS-207. GVAX is an allogeneic whole cell vaccine developed from pancreatic cancer cell lines. These cancer cells are irradiated, to prevent them from dividing and are genetically modified to secrete GM-CSF (Granulocyte Macrophage Colony Stimulating Factor). GM-CSF is important for the growth and activation of dendritic cells also known as Antigen Presenting Cells. This vaccine when injected attracts the dendritic cells to the vaccine injection site and the dendritic cells in turn, pick up the antigens from the vaccine and present them to the patient’s immune system. The immune system then mounts a response by activating tumor specific T-cells. This vaccine therefore theoretically boosts the body’s immune system to fight the patient’s tumor, without causing collateral damage. The second vaccine CRS-207 is live-attenuated (weakened) Listeria monocytogenes bacterium which expresses mesothelin and stimulates innate and adaptive immunity. It is genetically engineered to elicit an immune response against the tumor-associated antigen mesothelin, which has been shown to be expressed at higher levels on pancreatic cancer cells than on normal cells. Previous studies have demonstrated that survival can be improved by induction of mesothelin specific T-cell responses. In this study, 90 patients with metastatic pancreatic adenocarcinoma were randomly assigned in a 2:1 ratio to receive two doses of GVAX followed by four doses of CRS-207 or six doses of GVAX alone. Treatment was given every 3 weeks and low-dose CYTOXAN® (Cyclophosphamide) was given IV, the day before GVAX in both groups, to inhibit regulatory (suppressive) T-cells. More than 80% of the patients had at least one prior treatment for metastatic disease and 50% had two or more prior treatments. The primary endpoint was overall survival. Secondary endpoints included safety, clinical and immune responses. At a planned interim analysis, the median overall survival was 6.1 months with the combination of two vaccines vs 3.9 months with GVAX alone (HR=0.54, P=0.011), a 46% reduction in risk of death with the combination immunotherapy. The median overall survival in patients who received three total doses which included at least two doses of GVAX and at least one dose of CRS-207 was 9.7 months compared to 4.6 months for GVAX alone (HR=0.44, P=0.0074), a 56% reduction in the risk of death. In the subgroup of patients who had two or more prior chemotherapy regimens, combination immunotherapy given as third line therapy or greater resulted in a median overall survival of 5.1 months vs 3.7 months with GVAX alone (HR=0.34, P=0.001), a 66% reduction in risk of death. Stabilization of tumor marker CA19-9, was seen in 32% of patients receiving combination immunotherapy vs 13% in those who received GVAX alone (P=0.06). The one year survival probability doubled with the dual vaccine with an estimated one year survival of 24% for the combination immunotherapy group and 12% for the GVAX alone group. Toxicities included local reactions after GVAX and transient fevers, chills, and lymphopenia after CRS-207 administration. The authors concluded that immunotherapy with a combination of two vaccines improved overall survival in patients with metastatic pancreatic carcinoma, who had failed prior therapies. Le DT, Wang-Gillam A, Picozzi V, et al. J Clin Oncol 32, 2014 (suppl 3; abstr 177)
Circulating Tumor Cells and Response to Chemotherapy in Metastatic Breast Cancer SWOG S0500
SUMMARY: Circulating tumor cells (CTCs) are epithelial cells that are shed into the circulation from a primary or metastatic tumor. After being shed, CTCs can remain in the circulation or undergo apoptosis. Evaluation of CTCs during the course of disease and treatment has prognostic value. Because of the very low concentrations of CTCs (1 CTC in the background of millions of normal hematopoietic cells) in the peripheral blood, different technologies have been developed that will allow enrichment and detection of these CTCs.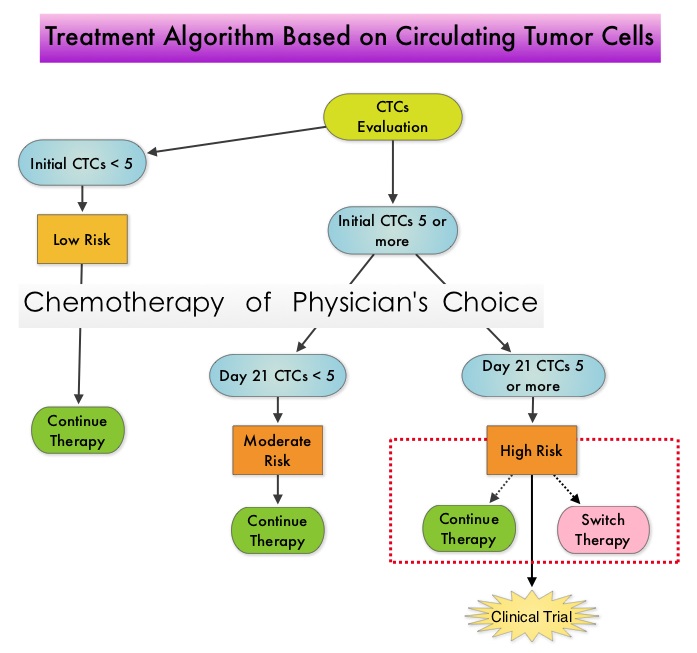 One such technology is the CellSearch® system which is the first FDA-approved test for CTC assessment, in the peripheral blood of Metastatic Breast Cancer (MBC) patients. This automated system is able to enrich the peripheral blood sample with CTCs and the cells then are fluorescently stained for CytoKeratins (CK8,18 and 19), Common Leukocyte Antigen (CD45) and a nuclear dye (DAPI). CTCs are identified when they are CK and DAPI positive and CD45 negative. In essence, CTC assessment, is a real time, peripheral blood evaluation (“Liquid Biopsy”) in MBC patients. Previously published studies have concluded that in patients with MBC, increased levels of CTCs prior to administration of a new therapy was associated with poor outcomes and failure of CTCs to drop to below 5 CTCs per 7.5 mL of peripheral blood at 3- 5 weeks after systemic therapy initiation, predicted worse Progression Free Survival (PFS) and Overall Survival (OS), compared to those who did not have increased CTCs at baseline or had increased CTCs at baseline and but not at 3-5 weeks after therapy. With this background, a randomized study was conducted to assess whether changing treatment after one cycle of first line chemotherapy, in those with a persistent increase in CTCs, improved OS. Evaluable patients were initially divided into two groups – Group A (N=276) included patients who did not have increased CTCs at baseline and Group B (N=288) included patients who had 5 or more CTCs per 7.5 mL of peripheral blood. Eligible patients were chemotherapy naïve for MBC and were treated with single agent chemotherapy. The choice of chemotherapy was at the discretion of the attending physician. Patients in Group A remained on initial therapy until disease progression whereas patients in Group B had CTC evaluation at Day 22 and those with decreased CTCs remained on the initial therapy (N=165). Patients who had persistently increased CTCs at Day 22 (N=123), were then randomly assigned to either continue the initial therapy (Group C1) or switch to a different chemotherapy regimen (Group C2). The median Overall Survival for Groups A, B, and C (C1 and C2 combined) were 35 months, 23 months, and 13 months, respectively (P <0.001). There was no difference in median Overall Survival between Groups C1 and C2 (10.7 and 12.5 months, respectively (P = 0.98). The authors concluded that CTCs in patients with Metastatic Breast Cancer receiving first line chemotherapy has significant prognostic value and changing to a different chemotherapy regimen based on persistently increased CTCs after 3 weeks of first line chemotherapy, had no impact in prolonging Overall Survival. This group of patients (C1 and C2) should be encouraged to enroll in clinical trials as standard chemotherapy may not be as effective. CTC count can prognosticate Progression Free Survival and Overall Survival early in the treatment course thereby allowing customized care. Further, CTC enumeration, unlike mucin based serum biomarkers such as CEA and CA15-3, better correlates with clinical and pathological characteristics of the disease. Smerage JB, Barlow WE, Hortobagyi GN, et al. DOI: 10.1200/JCO.2014.56.2561
One such technology is the CellSearch® system which is the first FDA-approved test for CTC assessment, in the peripheral blood of Metastatic Breast Cancer (MBC) patients. This automated system is able to enrich the peripheral blood sample with CTCs and the cells then are fluorescently stained for CytoKeratins (CK8,18 and 19), Common Leukocyte Antigen (CD45) and a nuclear dye (DAPI). CTCs are identified when they are CK and DAPI positive and CD45 negative. In essence, CTC assessment, is a real time, peripheral blood evaluation (“Liquid Biopsy”) in MBC patients. Previously published studies have concluded that in patients with MBC, increased levels of CTCs prior to administration of a new therapy was associated with poor outcomes and failure of CTCs to drop to below 5 CTCs per 7.5 mL of peripheral blood at 3- 5 weeks after systemic therapy initiation, predicted worse Progression Free Survival (PFS) and Overall Survival (OS), compared to those who did not have increased CTCs at baseline or had increased CTCs at baseline and but not at 3-5 weeks after therapy. With this background, a randomized study was conducted to assess whether changing treatment after one cycle of first line chemotherapy, in those with a persistent increase in CTCs, improved OS. Evaluable patients were initially divided into two groups – Group A (N=276) included patients who did not have increased CTCs at baseline and Group B (N=288) included patients who had 5 or more CTCs per 7.5 mL of peripheral blood. Eligible patients were chemotherapy naïve for MBC and were treated with single agent chemotherapy. The choice of chemotherapy was at the discretion of the attending physician. Patients in Group A remained on initial therapy until disease progression whereas patients in Group B had CTC evaluation at Day 22 and those with decreased CTCs remained on the initial therapy (N=165). Patients who had persistently increased CTCs at Day 22 (N=123), were then randomly assigned to either continue the initial therapy (Group C1) or switch to a different chemotherapy regimen (Group C2). The median Overall Survival for Groups A, B, and C (C1 and C2 combined) were 35 months, 23 months, and 13 months, respectively (P <0.001). There was no difference in median Overall Survival between Groups C1 and C2 (10.7 and 12.5 months, respectively (P = 0.98). The authors concluded that CTCs in patients with Metastatic Breast Cancer receiving first line chemotherapy has significant prognostic value and changing to a different chemotherapy regimen based on persistently increased CTCs after 3 weeks of first line chemotherapy, had no impact in prolonging Overall Survival. This group of patients (C1 and C2) should be encouraged to enroll in clinical trials as standard chemotherapy may not be as effective. CTC count can prognosticate Progression Free Survival and Overall Survival early in the treatment course thereby allowing customized care. Further, CTC enumeration, unlike mucin based serum biomarkers such as CEA and CA15-3, better correlates with clinical and pathological characteristics of the disease. Smerage JB, Barlow WE, Hortobagyi GN, et al. DOI: 10.1200/JCO.2014.56.2561
Improved Survival with Bevacizumab in Advanced Cervical Cancer
SUMMARY: Cervical cancer is the fourth most common cancer affecting women, worldwide. It is also the fourth most common cause of cancer death. With the availability of widespread screening techniques and HPV vaccination in the U.S., the incidence of cervical cancer is declining. Treatment of advanced cervical cancer continues to be a challenge. The FDA recently approved AVASTIN® (Bevacizumab) for the treatment of persistent, recurrent or metastatic cervical cancer, in combination with TAXOL® (Paclitaxel) and Cisplatin or TAXOL® and HYCAMTIN® (Topotecan). The approval was based on the results of an international, randomized, four-arm, 2×2 factorial design trial with two primary comparisons of Overall Survival (OS). The first comparison was between AVASTIN® plus chemotherapy versus chemotherapy alone. The second comparison of OS was between the platinum doublet versus the non-platinum doublet chemotherapy irrespective of addition of AVASTIN®. AVASTIN®, a humanized Vascular Endothelial Growth Factor (VEGF) targeted monoclonal antibody, has demonstrated single-agent activity in heavily pretreated patients with recurrent cervical carcinoma in phase II trials. In this randomized study, 452 enrolled patients with metastatic, persistent or recurrent cervical cancer received one of the four treatment regimens using a 2×2 factorial design. The four treatment groups included a) Cisplatin 50 mg/m2 plus TAXOL® (Paclitaxel) 135 or 175 mg/m2 (N=114), b) HYCAMTIN® (Topotecan) 0.75 mg/m2 given on D1 thru D3 plus TAXOL® 175 mg/m2 given on Day 1 (N=111), c) Cisplatin 50 mg/m2 plus TAXOL® 135 or 175 mg/m2 given along with AVASTIN® 15mg/kg on Day 1 (N=115) and d) HYCAMTIN® 0.75 mg/m2 given on D1 thru D3 plus TAXOL® 175 mg/m2 given on Day 1, along with AVASTIN® 15mg/kg on day 1 (N=112). Treatment was given every 21 days until disease progression, the development of unacceptable toxicities or a complete response was noted. The primary end point was Overall Survival and secondary endpoints included Progression Free Survival (PFS) and Response Rate (RR). When outcomes were analyzed, HYCAMTIN® based chemotherapy was not superior to Cisplatin based chemotherapy, regardless of prior exposure to Cisplatin. At a median follow-up of 20.8 months, the addition of AVASTIN® to chemotherapy resulted in a significantly longer median Overall Survival (17 vs 13.3 months; HR=0.71; P=0.004), significantly longer median PFS (8.2 vs 5.9 months; HR=0.67; P=0.002) and Response Rate (48% vs 36%; P=0.008), compared to combination chemotherapy alone. With regards to the second primary comparison of Overall Survival, the TAXOL® plus HYCAMTIN® with or without AVASTIN® groups did not demonstrate an improvement in Overall Survival compared to the TAXOL® plus Cisplatin with or without AVASTIN® groups. The benefit with added AVASTIN® was noted in all subgroups regardless of age, race, performance status and prior platinum exposure. Treatment was in general well tolerated without significant reduction in quality of life. As was seen in other tumor types, AVASTIN® based chemotherapy regimen was associated with a higher incidence of hypertension and thromboembolic events. The authors concluded that the addition of AVASTIN® to combination chemotherapy significantly decreased the risk of death in patients with recurrent, persistent, or metastatic cervical cancer. It can also be concluded from this study that the TAXOL® with HYCAMTIN® plus AVASTIN® is an acceptable alternative for women with advanced cervical cancer, who are not candidates for Cisplatin based chemotherapy. Tewari KS, Sill MW, Long HJ, et al. N Engl J Med 2014; 370:734-743
Confirmatory open-label, single-arm, multicenter phase 2 study of the BiTE antibody, Blinatumomab in patients (pts) with relapsed/refractory B-precursor acute lymphoblastic leukemia (r/r ALL)
SUMMARY:The FDA in July 2014, granted Breakthrough Therapy Designation to Blinatumomab, a bispecific T cell engager (BiTE) antibody, for adults with Philadelphia-negative (Ph-) Relapsed/Refractory B-precursor Acute Lymphoblastic Leukemia (ALL). BiTE® technology engages the body’s immune system to detect and target malignant cells. These modified antibodies are designed to engage two different targets simultaneously, thereby placing the T cells within reach of the targeted cancer cell and facilitating apoptosis of the cancer cell. BiTE antibodies are currently being investigated to treat a wide variety of malignancies. Blinatumomab is an investigational BiTE® antibody designed to direct the patients T cells against CD19, a protein found on the surface of B-cell derived leukemias and lymphomas.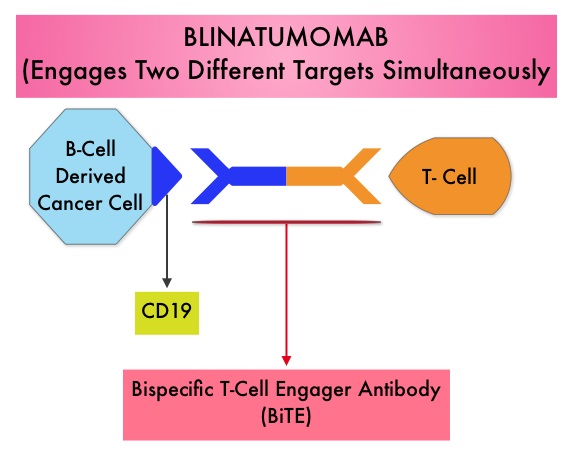 The Breakthrough Therapy Designation to Blinatumomab was based on a Phase II study in which 189 patients with Philadelphia chromosome negative ALL were enrolled. The median age was 39 years, and patients had their 1st relapse and were refractory to post hematopoietic stem cell transplantation less than 12 months before. About a third of the patients had at least 2 salvage therapies. Blinatumomab was given by continuous IV infusion, 4 weeks on and 2 weeks off for up to 5 cycles and the median number of cycles given were 2. The primary endpoint was complete remission (CR) or CR with partial hematological recovery (CRh) within the first 2 cycles of treatment. At the time of primary analysis, 43% of patients achieved a CR or CRh and 80% of responses occurred within cycle 1. Further, the Complete Remissions (CR) and CR with partial hematological recovery (CRh) were seen in all subgroups of patients, although this was more pronounced in those with less than 50% bone marrow blasts. The median Relapse Free Survival and Overall survival were 5.9 months and 6.1 months respectively. The most frequent grade 3 adverse events were febrile neutropenia, neutropenia, and anemia, occurring in 26%, 15%, and 15% of patients, respectively. The authors concluded that Blinatumomab has significant single agent antileukemia activity in a difficult-to-treat population with Relapsed and Refractory Acute Lymphoblastic Leukemia. Clinical trials will hopefully address whether Blinatumomab can serve as a bridge to transplantation, in patients with Relapsed and Refractory B-cell ALL. Topp MS, Goekbuget N, Stein AS, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 7005)</s
The Breakthrough Therapy Designation to Blinatumomab was based on a Phase II study in which 189 patients with Philadelphia chromosome negative ALL were enrolled. The median age was 39 years, and patients had their 1st relapse and were refractory to post hematopoietic stem cell transplantation less than 12 months before. About a third of the patients had at least 2 salvage therapies. Blinatumomab was given by continuous IV infusion, 4 weeks on and 2 weeks off for up to 5 cycles and the median number of cycles given were 2. The primary endpoint was complete remission (CR) or CR with partial hematological recovery (CRh) within the first 2 cycles of treatment. At the time of primary analysis, 43% of patients achieved a CR or CRh and 80% of responses occurred within cycle 1. Further, the Complete Remissions (CR) and CR with partial hematological recovery (CRh) were seen in all subgroups of patients, although this was more pronounced in those with less than 50% bone marrow blasts. The median Relapse Free Survival and Overall survival were 5.9 months and 6.1 months respectively. The most frequent grade 3 adverse events were febrile neutropenia, neutropenia, and anemia, occurring in 26%, 15%, and 15% of patients, respectively. The authors concluded that Blinatumomab has significant single agent antileukemia activity in a difficult-to-treat population with Relapsed and Refractory Acute Lymphoblastic Leukemia. Clinical trials will hopefully address whether Blinatumomab can serve as a bridge to transplantation, in patients with Relapsed and Refractory B-cell ALL. Topp MS, Goekbuget N, Stein AS, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 7005)</s
KEYTRUDA® – A promising Immunotherapy for Metastatic Melanoma
The FDA granted accelerated approval to KEYTRUDA® (Pembrolizumab), a humanized anti PD-1 antibody, for the treatment of patients with advanced Metastatic Melanoma, who have disease progression following YERVOY® (Ipilimumab) and if BRAF V600 mutation positive, a BRAF inhibitor. KEYTRUDA® produced significant and durable responses in patients with advanced Melanoma, regardless of prior therapy with YERVOY® and this benefit was accomplished with minimal toxicities. This new entry will revolutionize the treatment of advanced Melanoma.
