SUMMARY: The FDA has granted Breakthrough Therapy Designation to immunotherapy with CTL019, which are genetically engineered T-cells. Chimeric Antigen Receptor (CAR) T-cell therapy is a type of immunotherapy in which T cells collected from the patient’s own blood and are genetically engineered to produce special receptors on their surface called chimeric antigen receptors (CAR’s). The cytotoxic T cells with these chimeric antigen receptors on their surface are now able to recognize a specific antigen on tumor cells. These engineered CAR T-cells which are grown in the lab are then infused into the patient and they in turn proliferate in the patient’s body and the engineered receptor on their surface help recognize and kill cancer cells that expresses that specific antigen. CTL019 are genetically engineered T-cells using CAR technology that seeks out cancer cells expressing the antigen CD19, which is found uniquely on B cells and destroy them. Patients, following treatment with CAR T-cells, develop B-cell aplasia (absence of CD19 positive cells) due to B-cell destruction and may need immunoglobin replacement. Hence, B-cell aplasia can be a useful therapeutic marker, as continued B-cell aplasia has been seen in all patients who had sustained remission, following CAR T-cell therapy. Cytokine Release Syndrome, an inflammatory process is the most common and serious side effect of CAR T-cell therapy and is associated with marked elevation of Interleukin-6. Cytokine release is important for T-cell activation and can result in high fevers and myalgias. This is usually self limiting although if severe can be associated with hypotension and respiratory insufficiency. Tocilizumab, an Interleukin-6 receptor blocking antibody produces a rapid improvement in symptoms. This is however not recommended unless the symptoms are severe and life threatening, as blunting the cytokine response can in turn negate T-cell proliferation. Elevated serum Ferritin and C-reactive protein levels are surrogate markers for severe Cytokine Release Syndrome. The CAR T-cells have been shown to also access sanctuary sites such as the central nervous system and eradicate cancer cells. CD19 antigen is expressed by majority of the B cell malignancies and therefore most studies using CAR T-cell therapy have focused on the treatment of advanced B-cell malignancies such as Chronic Lymphocytic Leukemia (CLL), Acute Lymphoblastic Leukemia (ALL) and Non Hodgkin lymphoma (NHL), such as Diffuse Large B-Cell Lymphoma (DLBCL). Previously published studies have shown significant responses with CAR T-cell therapy in patients with relapsed and refractory B-cell ALL. But the durability of remission has remained unclear.
Patients, following treatment with CAR T-cells, develop B-cell aplasia (absence of CD19 positive cells) due to B-cell destruction and may need immunoglobin replacement. Hence, B-cell aplasia can be a useful therapeutic marker, as continued B-cell aplasia has been seen in all patients who had sustained remission, following CAR T-cell therapy. Cytokine Release Syndrome, an inflammatory process is the most common and serious side effect of CAR T-cell therapy and is associated with marked elevation of Interleukin-6. Cytokine release is important for T-cell activation and can result in high fevers and myalgias. This is usually self limiting although if severe can be associated with hypotension and respiratory insufficiency. Tocilizumab, an Interleukin-6 receptor blocking antibody produces a rapid improvement in symptoms. This is however not recommended unless the symptoms are severe and life threatening, as blunting the cytokine response can in turn negate T-cell proliferation. Elevated serum Ferritin and C-reactive protein levels are surrogate markers for severe Cytokine Release Syndrome. The CAR T-cells have been shown to also access sanctuary sites such as the central nervous system and eradicate cancer cells. CD19 antigen is expressed by majority of the B cell malignancies and therefore most studies using CAR T-cell therapy have focused on the treatment of advanced B-cell malignancies such as Chronic Lymphocytic Leukemia (CLL), Acute Lymphoblastic Leukemia (ALL) and Non Hodgkin lymphoma (NHL), such as Diffuse Large B-Cell Lymphoma (DLBCL). Previously published studies have shown significant responses with CAR T-cell therapy in patients with relapsed and refractory B-cell ALL. But the durability of remission has remained unclear.
The authors in this study, treated a total of 30 patients with relapsed or refractory ALL ( included those who had relapsed after allogeneic stem cell transplantation and those refractory to CD19 directed bispecific antibody Blinatumomab), with autologous Chimeric Antigen Receptor (CAR) T-cells (CTL019 T-cells) and monitored response rates, toxicities as well as proliferation and persistence of circulating CTL019 T-cells in the patient’s body. The first assessment was performed 1 month after infusion of CTL019 and 90% of the patients were in complete remission and sustained remissions were noted for up to 2 years. At a median follow up of 6 months, the event free survival was 67% and overall survival was 78%. The authors compared this efficacy data with the FDA approved agents for relapsed ALL such as Clofarabine, Nelarabine and Liposomal encapsulated Vincristine, which have a complete remission of less than 25% with a median duration of response of 4-9 weeks. Persisting CTL019 T-cells in the body is a marker of therapeutic efficacy. CTL019 T-cells proliferated in the patient’s body and was detectable in the blood bone marrow, and cerebrospinal fluid of patients who had a response. At 6 months, the probability that a patient would have persistence of CTL019 T-cells was 68% and the probability that a patient would have relapse free B-cell aplasia was 73%. Severe Cytokine Release Syndrome was noted in 27% of the patients and these patients had a higher disease burden before CTL019 infusion. All of these patients were effectively treated with the Interleukin-6 receptor blocking antibody Tocilizumab. The authors concluded that Chimeric Antigen Receptor modified T-cell therapy against CD19 positive cells (CTL019) was highly efficacious, in patients with relapsed and refractory ALL and was associated with a high and durable remission rate. This technology may be applied to other malignancies, as new antigen targets are identified. Maude SL, Frey N, Shaw PA, et al. N Engl J Med 2014; 371:1507-1517

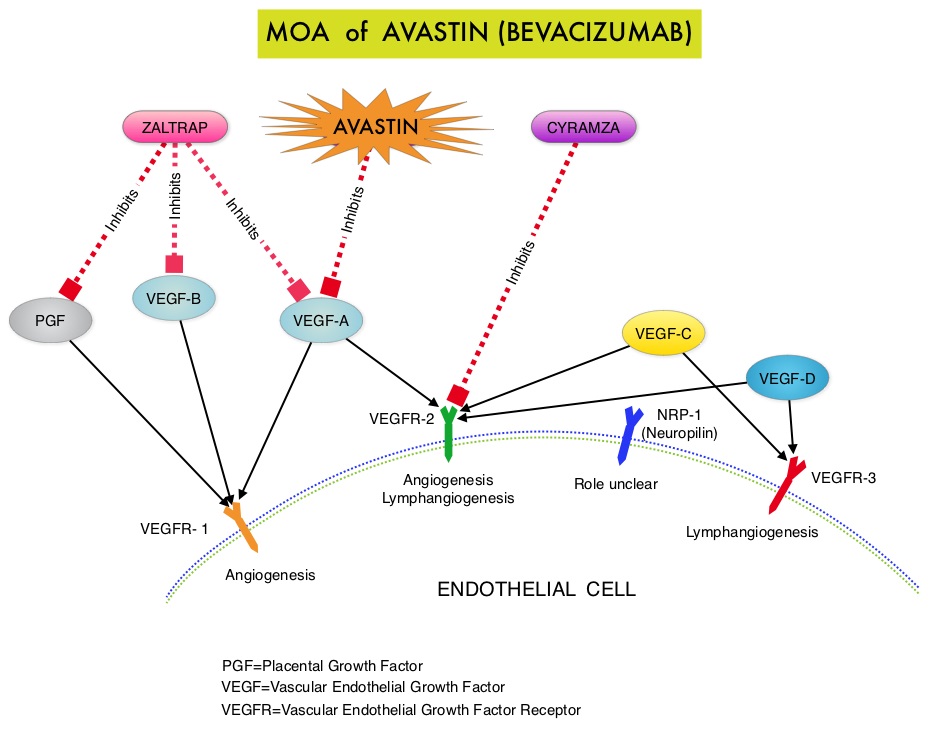 The primary end point was Progression Free Survival (PFS) and secondary end points included Objective Response Rate (ORR), Overall Survival (OS), safety, and patient reported outcomes. The combination of AVASTIN® plus chemotherapy resulted in a 62% reduction in the risk of progression compared to those who received chemotherapy alone, with a median PFS of 6.8 months for the AVASTIN® plus chemotherapy group versus 3.4 months for the single agent chemotherapy group (HR=0.38, P<0.0001) and thus met the primary endpoint of this clinical trial. This PFS benefit was seen consistently across all subgroups including the subgroup of patients with ascites. The ORR was 27.3% with the AVASTIN® combination versus 11.8% with single agent chemotherapy (P =0.001). The median OS was 16.6 months for the AVASTIN® combination versus 13.3 months for the single agent chemotherapy group (HR=0.85; P < .17). The lack of statistical significance in the OS has been attributed to cross over of 40% of patients, initially randomized to the chemotherapy alone group, who upon progression received AVASTIN®. There was a 15% improvement in abdominal and GI symptoms as reported by patients, with the AVASTIN® combination, compared to chemotherapy alone. On exploratory analyses it was noted that the addition of AVASTIN® to TAXOL® resulted in the most benefit, with a 5.7 month improvement in median PFS (9.6 versus 3.9 months), a 23% improvement in the overall response rate (53% versus 30%) and a 9.2 month improvement in median OS (22.4 versus 13.2 months) compared to single agent TAXOL®. This benefit was seen in spite of the fact that 97% of the patients in the TAXOL® group had received this agent with previous chemotherapy regimens. These findings suggest that patients who have received prior treatment with TAXOL® may benefit from AVASTIN® plus weekly TAXOL®. The most common adverse reactions (greater than or equal to 15%) in patients treated with AVASTIN® plus chemotherapy were neutropenia, peripheral neuropathy, hypertension and GI perforation occurred in 1.7% of these patients. This low perforation rate has been attributed to the exclusion of patients with rectosigmoid involvement by pelvic examination or bowel involvement on CT scan as well as those with clinical symptoms of bowel obstruction. The authors concluded that AVASTIN® in combination with chemotherapy significantly improved Progression Free Survival and Objective Response Rates in patients with Platinum Resistant Recurrent Ovarian Cancer. Pujade-Lauraine E, Hilpert F, Weber B, et al. J Clin Oncol 2014;32:1302-1308
The primary end point was Progression Free Survival (PFS) and secondary end points included Objective Response Rate (ORR), Overall Survival (OS), safety, and patient reported outcomes. The combination of AVASTIN® plus chemotherapy resulted in a 62% reduction in the risk of progression compared to those who received chemotherapy alone, with a median PFS of 6.8 months for the AVASTIN® plus chemotherapy group versus 3.4 months for the single agent chemotherapy group (HR=0.38, P<0.0001) and thus met the primary endpoint of this clinical trial. This PFS benefit was seen consistently across all subgroups including the subgroup of patients with ascites. The ORR was 27.3% with the AVASTIN® combination versus 11.8% with single agent chemotherapy (P =0.001). The median OS was 16.6 months for the AVASTIN® combination versus 13.3 months for the single agent chemotherapy group (HR=0.85; P < .17). The lack of statistical significance in the OS has been attributed to cross over of 40% of patients, initially randomized to the chemotherapy alone group, who upon progression received AVASTIN®. There was a 15% improvement in abdominal and GI symptoms as reported by patients, with the AVASTIN® combination, compared to chemotherapy alone. On exploratory analyses it was noted that the addition of AVASTIN® to TAXOL® resulted in the most benefit, with a 5.7 month improvement in median PFS (9.6 versus 3.9 months), a 23% improvement in the overall response rate (53% versus 30%) and a 9.2 month improvement in median OS (22.4 versus 13.2 months) compared to single agent TAXOL®. This benefit was seen in spite of the fact that 97% of the patients in the TAXOL® group had received this agent with previous chemotherapy regimens. These findings suggest that patients who have received prior treatment with TAXOL® may benefit from AVASTIN® plus weekly TAXOL®. The most common adverse reactions (greater than or equal to 15%) in patients treated with AVASTIN® plus chemotherapy were neutropenia, peripheral neuropathy, hypertension and GI perforation occurred in 1.7% of these patients. This low perforation rate has been attributed to the exclusion of patients with rectosigmoid involvement by pelvic examination or bowel involvement on CT scan as well as those with clinical symptoms of bowel obstruction. The authors concluded that AVASTIN® in combination with chemotherapy significantly improved Progression Free Survival and Objective Response Rates in patients with Platinum Resistant Recurrent Ovarian Cancer. Pujade-Lauraine E, Hilpert F, Weber B, et al. J Clin Oncol 2014;32:1302-1308
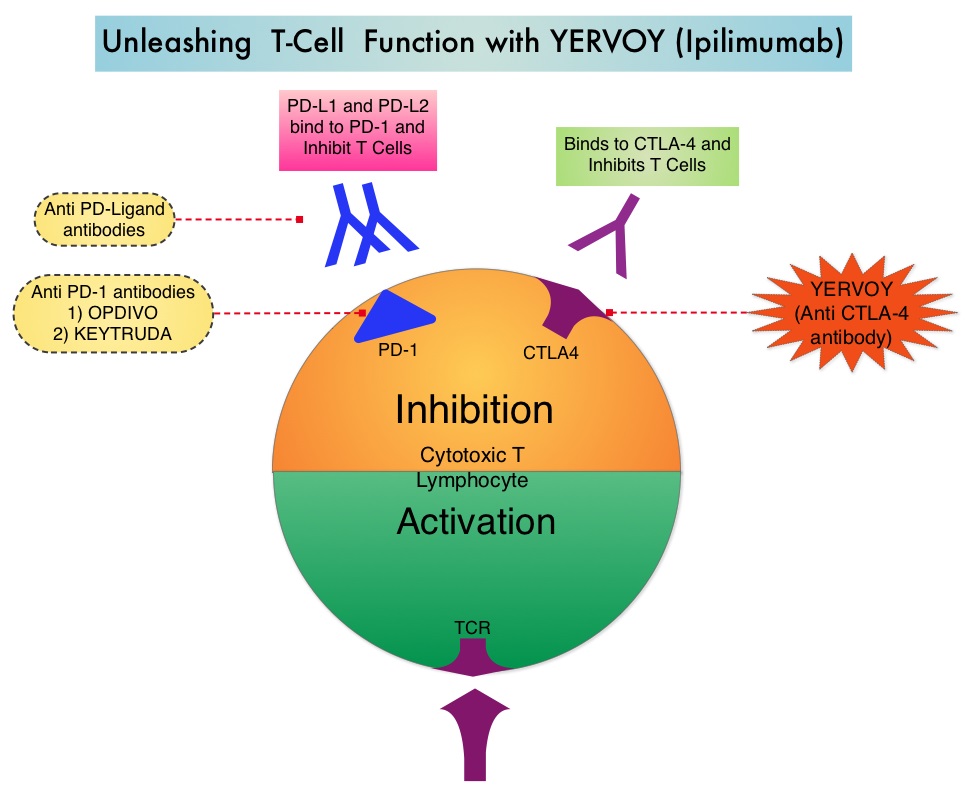 Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent, related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. The authors in this randomized study, compared the efficacy of YERVOY® (Ipilimumab) plus Sargramostim with YERVOY® alone, for treatment of metastatic melanoma. The rationale for this study was based on the synergy that was noted between YERVOY® and GM-CSF in preclinical models. The first immune checkpoint protein to be clinically targeted was CTLA-4. YERVOY® is a fully human IgG1monoclonal antibody that blocks Immune checkpoint protein/receptor CTLA- 4 and counteracts immune regulatory cells. YERVOY® has been shown to prolong overall survival in patients with previously treated, unresectable or metastatic melanoma. GM-CSF is a cytokine that enhances the antitumor activity of T and B lymphocytes by activating the antigen presenting dendritic cells and recruiting macrophages. It however can induce negative regulatory immune responses.
Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent, related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. The authors in this randomized study, compared the efficacy of YERVOY® (Ipilimumab) plus Sargramostim with YERVOY® alone, for treatment of metastatic melanoma. The rationale for this study was based on the synergy that was noted between YERVOY® and GM-CSF in preclinical models. The first immune checkpoint protein to be clinically targeted was CTLA-4. YERVOY® is a fully human IgG1monoclonal antibody that blocks Immune checkpoint protein/receptor CTLA- 4 and counteracts immune regulatory cells. YERVOY® has been shown to prolong overall survival in patients with previously treated, unresectable or metastatic melanoma. GM-CSF is a cytokine that enhances the antitumor activity of T and B lymphocytes by activating the antigen presenting dendritic cells and recruiting macrophages. It however can induce negative regulatory immune responses. In this phase II randomized clinical trial conducted by the Eastern Cooperative Oncology Group (ECOG), patients with unresectable stage III or IV melanoma (N = 245), who had received at least 1 prior therapy and with no central nervous system metastases were randomized to receive either YERVOY® along with Sargramostim (N=123) or YERVOY® alone (N=122). Patients in the combination group (Group A) received YERVOY®10 mg/kg, IV on day 1 along with Sargramostim 250 μg given subcutaneously, on days 1 thru 14 of a 21day cycle, every 3 weeks for four cycles followed by YERVOY® maintenance every 12 weeks. Patients in Group B received YERVOY® alone. Treatment was continued until disease progression or uncontrolled toxicities. The primary endpoint was comparison of length of Overall Survival (OS). Secondary end points included Progression Free Survival (PFS), response rate, safety, and tolerability. With a median follow up of 13.3 months, the median OS for the combination of YERVOY® plus Sargramostim was 17.5 months vs 12.7 months for YERVOY® alone. The one year survival rate for YERVOY® plus Sargramostim was 68.9% compared to 52.9% for YERVOY® alone (HR=0.64; P=0.01). The median PFS was similar and was 3.1 months in both study groups. The explanation for similar PFS in both treatment groups may be due to both YERVOY® and Sargramostim bringing about inflammatory changes at the tumor sites, which in turn could be misinterpreted as disease progression, on radiological studies. The authors commented that PFS may not be an appropriate endpoint in immunotherapy trials. Grade 3 to 5 adverse events were less in the combination group (44.9%) compared to 58% for single agent YERVOY® (P=0.04). The authors concluded that treatment of unresectable stage III or IV melanoma patients with YERVOY® plus Sargramostim resulted in significantly longer overall survival with lower toxicities, compared to YERVOY® alone. Hodi SF, Lee S, McDermott DF, et al. JAMA 2014;312:1744-1753
In this phase II randomized clinical trial conducted by the Eastern Cooperative Oncology Group (ECOG), patients with unresectable stage III or IV melanoma (N = 245), who had received at least 1 prior therapy and with no central nervous system metastases were randomized to receive either YERVOY® along with Sargramostim (N=123) or YERVOY® alone (N=122). Patients in the combination group (Group A) received YERVOY®10 mg/kg, IV on day 1 along with Sargramostim 250 μg given subcutaneously, on days 1 thru 14 of a 21day cycle, every 3 weeks for four cycles followed by YERVOY® maintenance every 12 weeks. Patients in Group B received YERVOY® alone. Treatment was continued until disease progression or uncontrolled toxicities. The primary endpoint was comparison of length of Overall Survival (OS). Secondary end points included Progression Free Survival (PFS), response rate, safety, and tolerability. With a median follow up of 13.3 months, the median OS for the combination of YERVOY® plus Sargramostim was 17.5 months vs 12.7 months for YERVOY® alone. The one year survival rate for YERVOY® plus Sargramostim was 68.9% compared to 52.9% for YERVOY® alone (HR=0.64; P=0.01). The median PFS was similar and was 3.1 months in both study groups. The explanation for similar PFS in both treatment groups may be due to both YERVOY® and Sargramostim bringing about inflammatory changes at the tumor sites, which in turn could be misinterpreted as disease progression, on radiological studies. The authors commented that PFS may not be an appropriate endpoint in immunotherapy trials. Grade 3 to 5 adverse events were less in the combination group (44.9%) compared to 58% for single agent YERVOY® (P=0.04). The authors concluded that treatment of unresectable stage III or IV melanoma patients with YERVOY® plus Sargramostim resulted in significantly longer overall survival with lower toxicities, compared to YERVOY® alone. Hodi SF, Lee S, McDermott DF, et al. JAMA 2014;312:1744-1753
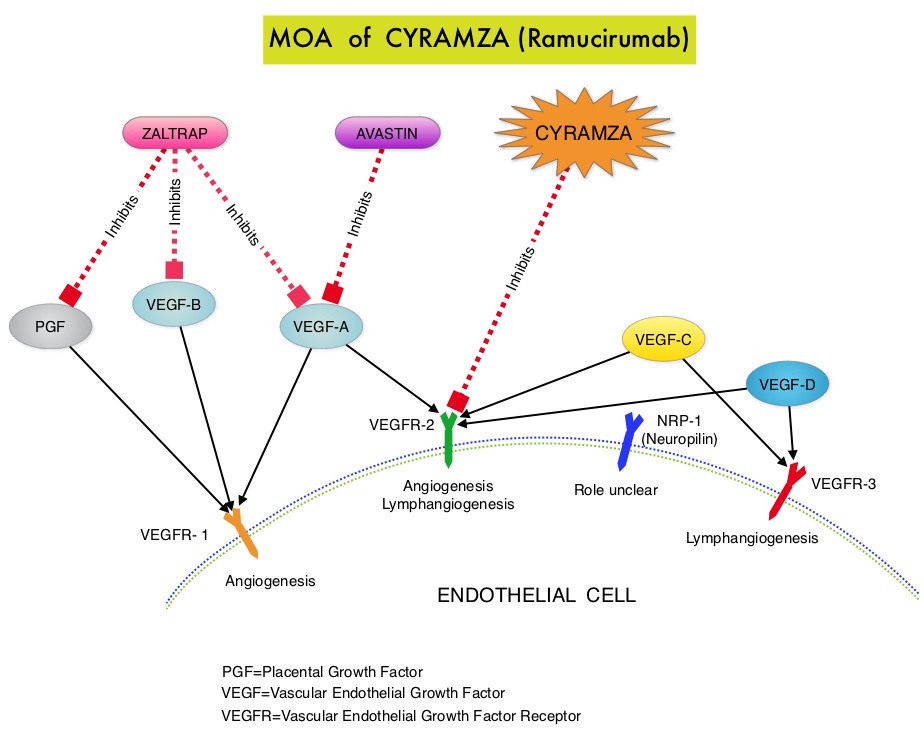 The RAINBOW study is an international, placebo-controlled, double-blind, phase III trial in which 665 patients with metastatic gastroesophageal junction or gastric adenocarcinoma, who had disease progression on or within 4 months after first-line platinum and fluoropyrimidine-based combination therapy, were included. Patients were randomly assigned to receive TAXOL® (Paclitaxel) 80 mg/m2 given on D1, 8, 15 along with Placebo (N=335) or the same dose and schedule of TAXOL® given along with CYRAMZA® at 8 mg/kg IV every 2 weeks (N=330), of a 28 day cycle. Treatment was continued until disease progression or unacceptable toxicities were noted. The primary endpoint was Overall Survival (OS). Secondary endpoints included Progression Free Survival (PFS), Objective Response Rate (ORR) and Time To Progression (TTP). The median OS for the combination of CYRAMZA® and TAXOL® was 9.6 months compared to 7.4 months for Placebo and TAXOL® (HR=0.81; P=0.017), resulting in a 19% reduction in the risk of death with the CYRAMZA® and TAXOL® combination. The secondary endpoints favored the CYRAMZA® and TAXOL® combination as well. The median PFS was 4.4 months and 2.9 months (HR=0.64; P<0.001), ORR was 28% and 16% (P<0.0001) and median TTP was 5.5 months and 3 months with the CYRAMZA® and TAXOL® combination vs Placebo and TAXOL® combination respectively. As one would expect, treatment related adverse events were seen more frequently in the CYRAMZA® and TAXOL® combination group. Significant were neutropenia, hypertension, fatigue and asthenia, diarrhea and epistaxis. The incidence of febrile neutropenia in the two treatment groups was however comparable (3.1% vs 2.4%). The authors concluded that the combination of CYRAMZA® and TAXOL® combination significantly improved both Progression Free and Overall Survival and also resulted in significantly improved disease control rates, in patients with metastatic gastroesophageal junction or gastric adenocarcinoma. Wilke H, Van Cutsem E, Oh SC, et al. J Clin Oncol 32, 2014 (suppl 3; abstr LBA7)
The RAINBOW study is an international, placebo-controlled, double-blind, phase III trial in which 665 patients with metastatic gastroesophageal junction or gastric adenocarcinoma, who had disease progression on or within 4 months after first-line platinum and fluoropyrimidine-based combination therapy, were included. Patients were randomly assigned to receive TAXOL® (Paclitaxel) 80 mg/m2 given on D1, 8, 15 along with Placebo (N=335) or the same dose and schedule of TAXOL® given along with CYRAMZA® at 8 mg/kg IV every 2 weeks (N=330), of a 28 day cycle. Treatment was continued until disease progression or unacceptable toxicities were noted. The primary endpoint was Overall Survival (OS). Secondary endpoints included Progression Free Survival (PFS), Objective Response Rate (ORR) and Time To Progression (TTP). The median OS for the combination of CYRAMZA® and TAXOL® was 9.6 months compared to 7.4 months for Placebo and TAXOL® (HR=0.81; P=0.017), resulting in a 19% reduction in the risk of death with the CYRAMZA® and TAXOL® combination. The secondary endpoints favored the CYRAMZA® and TAXOL® combination as well. The median PFS was 4.4 months and 2.9 months (HR=0.64; P<0.001), ORR was 28% and 16% (P<0.0001) and median TTP was 5.5 months and 3 months with the CYRAMZA® and TAXOL® combination vs Placebo and TAXOL® combination respectively. As one would expect, treatment related adverse events were seen more frequently in the CYRAMZA® and TAXOL® combination group. Significant were neutropenia, hypertension, fatigue and asthenia, diarrhea and epistaxis. The incidence of febrile neutropenia in the two treatment groups was however comparable (3.1% vs 2.4%). The authors concluded that the combination of CYRAMZA® and TAXOL® combination significantly improved both Progression Free and Overall Survival and also resulted in significantly improved disease control rates, in patients with metastatic gastroesophageal junction or gastric adenocarcinoma. Wilke H, Van Cutsem E, Oh SC, et al. J Clin Oncol 32, 2014 (suppl 3; abstr LBA7) The different types of external beam radiation treatments include 3-Dimensional Conformal Radiation Therapy (3D-CRT) meant to deliver radiation to very precisely shaped target areas, IMRT or Intensity Modulated Radiation Therapy which allows different areas of a tumor or nearby tissues to receive different doses of radiation, Image Guided Radiation Therapy (IGRT) which allows reduction in the planned volume of tissue to be treated as changes in a tumor size are noted during treatment, Stereotactic RadioSurgery (SRS) which can deliver one or more high doses of radiation to a small tumor, Stereotactic Body Radiation Therapy (SBRT) or CYBERKNIFE® which is similar to SRS but also takes the normal motion of the body into account while treating malignancies involving the lung and liver and Proton Beam therapy. Proton beams unlike Photons, enter the skin and travel through the tissues and deposit much of their energy at the end of their path (known as the Bragg peak) and deposit less energy along the way. This is unlike Photons which deposit energy all along the path through the tissues and the deposited dose decreases with increasing depth. As a result, with Proton beam therapy, normal tissues are exposed to less radiation compared with Photons. Despite this advantage, tissue heterogeneity such as organ motion, tumor volume changes during treatment can have a significant negative impact on target coverage for Proton beam therapy and can result in damage to the surrounding tissues and potential complications. The authors in this review discussed the clinical applications of Proton therapy in Adult and Pediatric malignancies. Pediatric patients with malignancies have greater benefit with Proton beam therapy, with a statistically significant lower risk of secondary malignancies and less damage to the developing tissues and organs, compared to Photon therapy (External Beam Radiation Therapy). This clinical benefit may be less so in adult malignancies in spite of superior dosimetry, compared to external beam radiation, as adults are less prone to secondary malignancies compared to children.
The different types of external beam radiation treatments include 3-Dimensional Conformal Radiation Therapy (3D-CRT) meant to deliver radiation to very precisely shaped target areas, IMRT or Intensity Modulated Radiation Therapy which allows different areas of a tumor or nearby tissues to receive different doses of radiation, Image Guided Radiation Therapy (IGRT) which allows reduction in the planned volume of tissue to be treated as changes in a tumor size are noted during treatment, Stereotactic RadioSurgery (SRS) which can deliver one or more high doses of radiation to a small tumor, Stereotactic Body Radiation Therapy (SBRT) or CYBERKNIFE® which is similar to SRS but also takes the normal motion of the body into account while treating malignancies involving the lung and liver and Proton Beam therapy. Proton beams unlike Photons, enter the skin and travel through the tissues and deposit much of their energy at the end of their path (known as the Bragg peak) and deposit less energy along the way. This is unlike Photons which deposit energy all along the path through the tissues and the deposited dose decreases with increasing depth. As a result, with Proton beam therapy, normal tissues are exposed to less radiation compared with Photons. Despite this advantage, tissue heterogeneity such as organ motion, tumor volume changes during treatment can have a significant negative impact on target coverage for Proton beam therapy and can result in damage to the surrounding tissues and potential complications. The authors in this review discussed the clinical applications of Proton therapy in Adult and Pediatric malignancies. Pediatric patients with malignancies have greater benefit with Proton beam therapy, with a statistically significant lower risk of secondary malignancies and less damage to the developing tissues and organs, compared to Photon therapy (External Beam Radiation Therapy). This clinical benefit may be less so in adult malignancies in spite of superior dosimetry, compared to external beam radiation, as adults are less prone to secondary malignancies compared to children.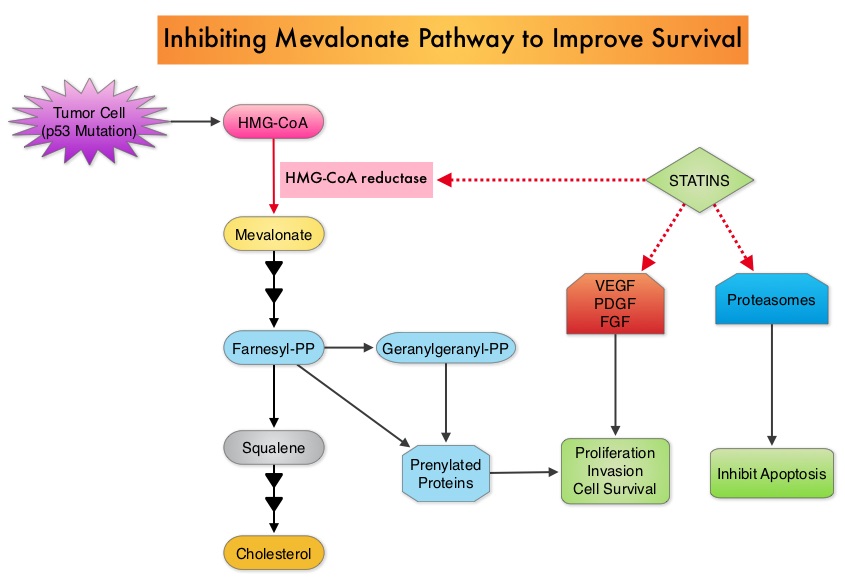 Statin use in cancer patients has been associated with a reduction in cancer related mortality in several clinical studies. This benefit has been attributed to the inhibition of HMG-CoA reductase, which is a rate limiting enzyme in the mevalonate and cholesterol synthesis pathway. The mevalonate pathway is upregulated by mutated p53 (tumor suppressor gene) which is often expressed in cancer cells. By inhibiting the mevalonate pathway, statins can reduce isoprenoid levels such as farnesylpyrophosphate (F-PP) and geranylgeranylpyrophosphate (GG-PP). These isoprenoids are essential for the posttranslational modification of several proteins involved in important intracellular signaling pathways and therefore play a crucial role in cell growth, proliferation, survival and migration. Statins also inhibit angiogenic pathways and proteasomes, thereby negatively impacting cell proliferation and survival. Survival benefit with statin use after colorectal cancer diagnosis has been unclear. To answer this question, the authors identified a cohort of patients (N=7657) diagnosed with stage I to III colorectal cancer from 1998 to 2009, in the National Cancer Data Repository (English Cancer Registry). Information on statin use was obtained from medical records of patients and in this cohort of patients 35% were identified to have used statin drugs following diagnosis of colorectal cancer. Twenty percent of these patients had stage I disease, 43% had stage II disease and 37% had stage III disease. Patients were followed up for 14 years following their diagnosis of colorectal cancer. Statin use after a diagnosis of colorectal cancer was associated with a 29% reduction in colorectal cancer-specific mortality (HR= 0.71). There was a dose-response association with a 36% reduction in colorectal cancer-specific mortality with statin use for more than 1 year (HR=0.64). Statin users after colorectal cancer diagnosis also had a 25% reduction in all-cause mortality (HR=0.75). The authors concluded that based on this large population based colorectal cancer cohort, statin use following diagnosis of colorectal cancer was associated with longer rates of survival. Cardwell CR, Hicks BM, Hughes C, et al. J Clin Oncol 2014;32:3177-3183
Statin use in cancer patients has been associated with a reduction in cancer related mortality in several clinical studies. This benefit has been attributed to the inhibition of HMG-CoA reductase, which is a rate limiting enzyme in the mevalonate and cholesterol synthesis pathway. The mevalonate pathway is upregulated by mutated p53 (tumor suppressor gene) which is often expressed in cancer cells. By inhibiting the mevalonate pathway, statins can reduce isoprenoid levels such as farnesylpyrophosphate (F-PP) and geranylgeranylpyrophosphate (GG-PP). These isoprenoids are essential for the posttranslational modification of several proteins involved in important intracellular signaling pathways and therefore play a crucial role in cell growth, proliferation, survival and migration. Statins also inhibit angiogenic pathways and proteasomes, thereby negatively impacting cell proliferation and survival. Survival benefit with statin use after colorectal cancer diagnosis has been unclear. To answer this question, the authors identified a cohort of patients (N=7657) diagnosed with stage I to III colorectal cancer from 1998 to 2009, in the National Cancer Data Repository (English Cancer Registry). Information on statin use was obtained from medical records of patients and in this cohort of patients 35% were identified to have used statin drugs following diagnosis of colorectal cancer. Twenty percent of these patients had stage I disease, 43% had stage II disease and 37% had stage III disease. Patients were followed up for 14 years following their diagnosis of colorectal cancer. Statin use after a diagnosis of colorectal cancer was associated with a 29% reduction in colorectal cancer-specific mortality (HR= 0.71). There was a dose-response association with a 36% reduction in colorectal cancer-specific mortality with statin use for more than 1 year (HR=0.64). Statin users after colorectal cancer diagnosis also had a 25% reduction in all-cause mortality (HR=0.75). The authors concluded that based on this large population based colorectal cancer cohort, statin use following diagnosis of colorectal cancer was associated with longer rates of survival. Cardwell CR, Hicks BM, Hughes C, et al. J Clin Oncol 2014;32:3177-3183 Evaluation of a patient with CUPS starts with gathering and incorporating medical information which includes the patient’s gender, medical history, clinical findings and sites of metastases. A CT scan of the chest, abdomen and pelvis with IV and oral contrast is recommended, although PET (Positron Emission Tomography) or an MRI can be performed in those with renal insufficiency or iodine allergy. PET scan is recommended for those with cervical lymphadenopathy with squamous histology, to help determine the extent of the disease and treatment planning for radiation. PET imaging is also helpful for patients with solitary metastases before locoregional therapies are planned, as well as assessing response in patients with predominantly bone only disease. In women presenting with isolated axillary lymphadenopathy, adenocarcinoma histology, negative mammograms and ultrasound, MRI of the breasts is indicated. With the exception of those patients with CUPS who present with cervical lymphadenopathy, diagnostic procedures such as bronchoscopy, EGD and colonoscopy are not recommended in asymptomatic patients. Tumor markers in general do not have diagnostic value in patients with CUPS although they could be utilized to monitor response to treatment. However, PSA when elevated in a male with adenocarcinoma and osteoblastic metastases, is suggestive of a prostate primary. Similarly an elevated Beta HCG and AFP in a patient with undifferentiated or poorly differentiated carcinoma, is suggestive of an extragonadal germ cell tumor and an elevated AFP is also helpful in making a diagnosis of Hepatoma. Approximately 60% of the patients with CUPS have well or moderately differentiated adenocarcinoma on light microscopy, 30% have poorly differentiated carcinoma or adenocarcinoma, 5% have poorly differentiated or undifferentiated malignancy and 5% have squamous cell carcinoma. Following histological evaluation on light microscopy, the biopsy specimen is further tested using ImmunoHistoChemical stains, using peroxidase labeled antibodies against tumor specific antigens, taking advantage of the similarities in the tumor profiles of primary and metastatic malignancies. After delineating a tumor as carcinoma, lymphoma, sarcoma or melanoma, additional IHC testing can help identify tumors such as a lung primary (postive Thyroid Transcription Factor 1-TTF1and positive CytoKeratin 7- CK7), lower gastrointestinal cancers (positive CK20, positive CDX2 and negative CK7) or a breast primary (positive CK7 and positive Mammaglobin). Tissue-of-Origin molecular profiling is based on the principle that in patients with CUPS, molecular signatures of metastatic tumors are similar to their primary tumor. Tissue-of-Origin molecular profiling is performed using tools such as DNA microarray, quantitative real time polymerase chain reaction assay (rt-PCR) or assays based on messenger RNA (mRNA) or microRNA. These tests are cost-effective and 70% – 90% accurate. This study can be performed on formalin-fixed samples as well as samples from fine needle aspiration. Even though platinum based chemotherapy has been the default regimen for patients with CUPS, histological evaluation of biopsy tissue by light microscopy, IHC testing and molecular profiling assay may complement each other and help guide the Health Care Provider to select site specific therapy. The survival outcomes of CUPS patients with a Tissue-of-Origin molecularly diagnosed profile are comparable to those with similar type advanced cancer with a known primary. The authors concluded that with additional molecular insights into tumor biology and availability of newer therapeutic agents, patients with CUPS and known primary tumors may eventually be treated alike. Varadhachary, GR and Raber, MN. N Engl J Med 2014; 371:757-765
Evaluation of a patient with CUPS starts with gathering and incorporating medical information which includes the patient’s gender, medical history, clinical findings and sites of metastases. A CT scan of the chest, abdomen and pelvis with IV and oral contrast is recommended, although PET (Positron Emission Tomography) or an MRI can be performed in those with renal insufficiency or iodine allergy. PET scan is recommended for those with cervical lymphadenopathy with squamous histology, to help determine the extent of the disease and treatment planning for radiation. PET imaging is also helpful for patients with solitary metastases before locoregional therapies are planned, as well as assessing response in patients with predominantly bone only disease. In women presenting with isolated axillary lymphadenopathy, adenocarcinoma histology, negative mammograms and ultrasound, MRI of the breasts is indicated. With the exception of those patients with CUPS who present with cervical lymphadenopathy, diagnostic procedures such as bronchoscopy, EGD and colonoscopy are not recommended in asymptomatic patients. Tumor markers in general do not have diagnostic value in patients with CUPS although they could be utilized to monitor response to treatment. However, PSA when elevated in a male with adenocarcinoma and osteoblastic metastases, is suggestive of a prostate primary. Similarly an elevated Beta HCG and AFP in a patient with undifferentiated or poorly differentiated carcinoma, is suggestive of an extragonadal germ cell tumor and an elevated AFP is also helpful in making a diagnosis of Hepatoma. Approximately 60% of the patients with CUPS have well or moderately differentiated adenocarcinoma on light microscopy, 30% have poorly differentiated carcinoma or adenocarcinoma, 5% have poorly differentiated or undifferentiated malignancy and 5% have squamous cell carcinoma. Following histological evaluation on light microscopy, the biopsy specimen is further tested using ImmunoHistoChemical stains, using peroxidase labeled antibodies against tumor specific antigens, taking advantage of the similarities in the tumor profiles of primary and metastatic malignancies. After delineating a tumor as carcinoma, lymphoma, sarcoma or melanoma, additional IHC testing can help identify tumors such as a lung primary (postive Thyroid Transcription Factor 1-TTF1and positive CytoKeratin 7- CK7), lower gastrointestinal cancers (positive CK20, positive CDX2 and negative CK7) or a breast primary (positive CK7 and positive Mammaglobin). Tissue-of-Origin molecular profiling is based on the principle that in patients with CUPS, molecular signatures of metastatic tumors are similar to their primary tumor. Tissue-of-Origin molecular profiling is performed using tools such as DNA microarray, quantitative real time polymerase chain reaction assay (rt-PCR) or assays based on messenger RNA (mRNA) or microRNA. These tests are cost-effective and 70% – 90% accurate. This study can be performed on formalin-fixed samples as well as samples from fine needle aspiration. Even though platinum based chemotherapy has been the default regimen for patients with CUPS, histological evaluation of biopsy tissue by light microscopy, IHC testing and molecular profiling assay may complement each other and help guide the Health Care Provider to select site specific therapy. The survival outcomes of CUPS patients with a Tissue-of-Origin molecularly diagnosed profile are comparable to those with similar type advanced cancer with a known primary. The authors concluded that with additional molecular insights into tumor biology and availability of newer therapeutic agents, patients with CUPS and known primary tumors may eventually be treated alike. Varadhachary, GR and Raber, MN. N Engl J Med 2014; 371:757-765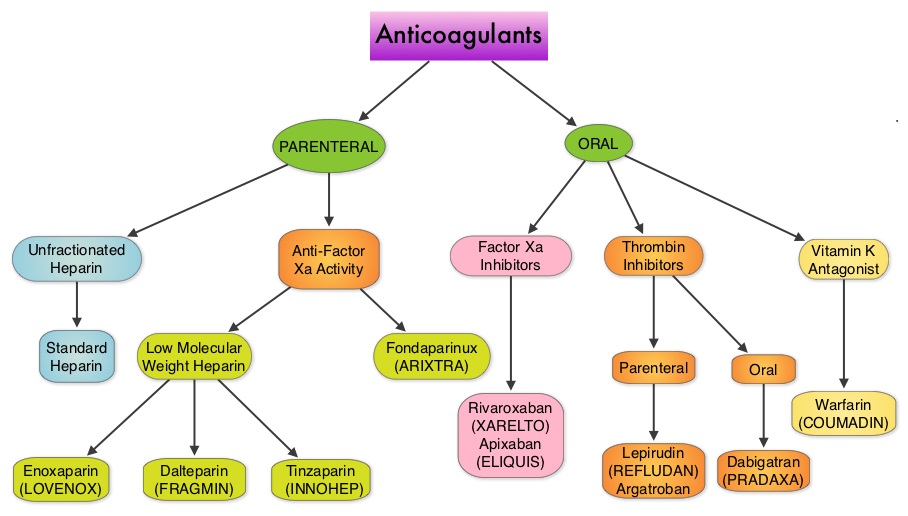 Even though Low Molecular Weight Heparin (LMWH) preparations as well as new oral anticoagulants have been available for the treatment Venous ThromboEmbolism, there has been very little guidance for Health Care Providers on the use of these newer agents. The authors in this analysis compared the efficacy and safety outcomes associated with different anticoagulation regimens for treatment of Venous ThromboEmbolism (VTE). These anticoagulant regimens included Unfractionated Heparin (UFH), Low Molecular Weight Heparin (LMWH) or Fondaparinux in combination with Vitamin K antagonists, LMWH with Dabigatran (PRADAXA®), Rivaroxaban (XARELTO®), Apixaban (ELIQUIS®) or Edoxaban and LMWH alone. This meta analysis included 44,989 patients from 45 randomized trials which reported rates of recurrent VTE and major bleeding in patients with acute VTE. In these Acute Deep Vein Thrombosis and Pulmonary Embolism trials, Rivaroxaban and Apixaban were evaluated without the use of initial LMWH whereas both Dabigatran or Edoxaban were assessed following an initial 5 day treatment with LMWH. This analysis was therefore able to assess clinical and safety outcomes associated with different anticoagulation regimens. The followings findings were noted:
Even though Low Molecular Weight Heparin (LMWH) preparations as well as new oral anticoagulants have been available for the treatment Venous ThromboEmbolism, there has been very little guidance for Health Care Providers on the use of these newer agents. The authors in this analysis compared the efficacy and safety outcomes associated with different anticoagulation regimens for treatment of Venous ThromboEmbolism (VTE). These anticoagulant regimens included Unfractionated Heparin (UFH), Low Molecular Weight Heparin (LMWH) or Fondaparinux in combination with Vitamin K antagonists, LMWH with Dabigatran (PRADAXA®), Rivaroxaban (XARELTO®), Apixaban (ELIQUIS®) or Edoxaban and LMWH alone. This meta analysis included 44,989 patients from 45 randomized trials which reported rates of recurrent VTE and major bleeding in patients with acute VTE. In these Acute Deep Vein Thrombosis and Pulmonary Embolism trials, Rivaroxaban and Apixaban were evaluated without the use of initial LMWH whereas both Dabigatran or Edoxaban were assessed following an initial 5 day treatment with LMWH. This analysis was therefore able to assess clinical and safety outcomes associated with different anticoagulation regimens. The followings findings were noted: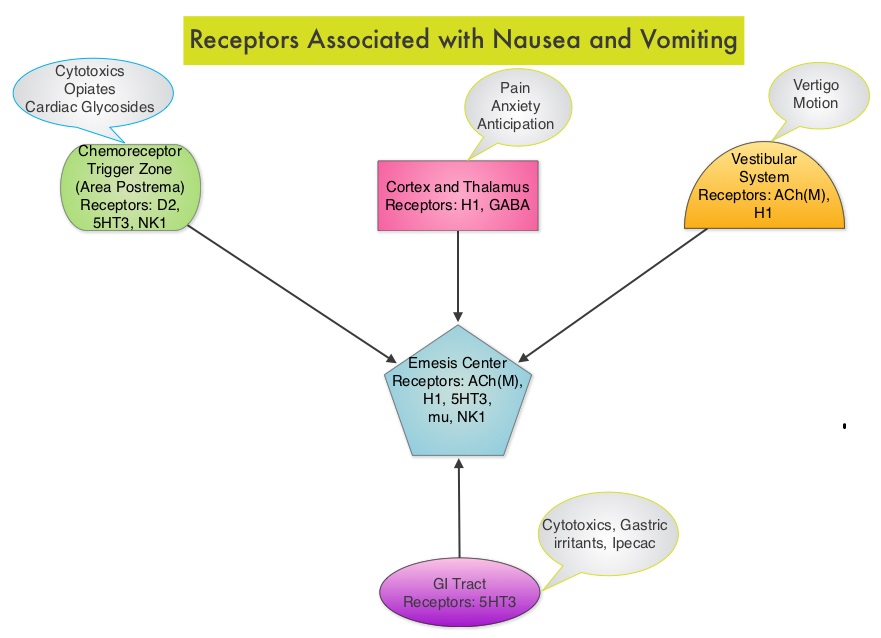 Acute CINV begins within the first 24 hours following chemotherapy administration, with most patients experiencing symptoms within the first four hours of treatment whereas delayed nausea and vomiting occurs more than 24 hours after chemotherapy administration and can persist for several days. Delayed CINV is often underestimated and a third of the patients receiving chemotherapy may experience delayed nausea and vomiting without prior acute nausea or vomiting. Acute nausea and vomiting is dependent on serotonin (5-hydroxytryptamine-5HT3) and its receptors. 5-HT3 receptors are located on vagal afferent pathway, which in turn activates the vomiting center to initiate the vomiting reflex. 5-HT3 receptors are located peripherally on the nerve endings of the vagus and centrally in the Chemoreceptor Trigger Zone of the area Postrema. Chemotherapeutic agents produce nausea and vomiting by stimulating the release of serotonin from the enterochromaffin cells of the small intestine. Delayed nausea and vomiting is associated with the activation of Neurokinin 1 (NK1) receptors by substance P. NK1 receptors are broadly distributed in the central and peripheral nervous systems. Netupitant inhibits substance P mediated responses. ALOXI® (Palonosetron) is a second generation 5-HT3 antagonist and has a 100 fold higher binding affinity to 5-HT3 receptor than other 5-HT3 receptor antagonists. AKYNZEO® (300 mg Netupitant/0.5 mg Palonosetron) is an oral, fixed combination product of Netupitant, a substance P/Neurokinin 1 (NK1) receptor antagonist, and ALOXI®, a serotonin (5- HT3) receptor antagonist.
Acute CINV begins within the first 24 hours following chemotherapy administration, with most patients experiencing symptoms within the first four hours of treatment whereas delayed nausea and vomiting occurs more than 24 hours after chemotherapy administration and can persist for several days. Delayed CINV is often underestimated and a third of the patients receiving chemotherapy may experience delayed nausea and vomiting without prior acute nausea or vomiting. Acute nausea and vomiting is dependent on serotonin (5-hydroxytryptamine-5HT3) and its receptors. 5-HT3 receptors are located on vagal afferent pathway, which in turn activates the vomiting center to initiate the vomiting reflex. 5-HT3 receptors are located peripherally on the nerve endings of the vagus and centrally in the Chemoreceptor Trigger Zone of the area Postrema. Chemotherapeutic agents produce nausea and vomiting by stimulating the release of serotonin from the enterochromaffin cells of the small intestine. Delayed nausea and vomiting is associated with the activation of Neurokinin 1 (NK1) receptors by substance P. NK1 receptors are broadly distributed in the central and peripheral nervous systems. Netupitant inhibits substance P mediated responses. ALOXI® (Palonosetron) is a second generation 5-HT3 antagonist and has a 100 fold higher binding affinity to 5-HT3 receptor than other 5-HT3 receptor antagonists. AKYNZEO® (300 mg Netupitant/0.5 mg Palonosetron) is an oral, fixed combination product of Netupitant, a substance P/Neurokinin 1 (NK1) receptor antagonist, and ALOXI®, a serotonin (5- HT3) receptor antagonist.  Taking advantage of the different mechanisms of action and synergy between these two agents, a randomized, double-blind, multinational study was conducted, comparing AKYNZEO® with ALOXI® in chemotherapy naive patients receiving anthracycline based chemotherapy regimens. One thousand four hundred and fifty five (N=1455) were randomized to receive either AKYNZEO® or ALOXI® and both groups received oral Dexamethasone as a part of their antiemetic regimen. The primary endpoint was complete response (CR) defined as no emesis, no rescue medication needed and no significant nausea. AKYNZEO® maintained superiority over ALOXI® for overall (0-120 hours) complete response and also maintained superiority over multiple chemotherapy cycles (P < 0.0001). The most common side effects for AKYNZEO® were headache, fatigue and constipation. The authors concluded that AKYNZEO®, by targeting dual antiemetic pathways, significantly improved chemotherapy induced nausea and vomiting compared to ALOXI® alone and this benefit was maintained over multiple cycles of moderately emetogenic chemotherapy. AKYNZEO® capsule can be administered as a single dose, one hour prior to the start of chemotherapy. Aapro MS, Karthaus M, Schwartzberg LS, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 9502)</s
Taking advantage of the different mechanisms of action and synergy between these two agents, a randomized, double-blind, multinational study was conducted, comparing AKYNZEO® with ALOXI® in chemotherapy naive patients receiving anthracycline based chemotherapy regimens. One thousand four hundred and fifty five (N=1455) were randomized to receive either AKYNZEO® or ALOXI® and both groups received oral Dexamethasone as a part of their antiemetic regimen. The primary endpoint was complete response (CR) defined as no emesis, no rescue medication needed and no significant nausea. AKYNZEO® maintained superiority over ALOXI® for overall (0-120 hours) complete response and also maintained superiority over multiple chemotherapy cycles (P < 0.0001). The most common side effects for AKYNZEO® were headache, fatigue and constipation. The authors concluded that AKYNZEO®, by targeting dual antiemetic pathways, significantly improved chemotherapy induced nausea and vomiting compared to ALOXI® alone and this benefit was maintained over multiple cycles of moderately emetogenic chemotherapy. AKYNZEO® capsule can be administered as a single dose, one hour prior to the start of chemotherapy. Aapro MS, Karthaus M, Schwartzberg LS, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 9502)</s