SUMMARY: The FDA on November 5, 2014 approved CYRAMZA® (Ramucirumab) for use in combination with TAXOL® (Paclitaxel) for the treatment of patients with advanced gastric or GastroEsophageal Junction (GEJ) adenocarcinoma. CYRAMZA® was approved by the FDA in April, 2014 as a single agent for the treatment of patients with advanced gastric or GEJ adenocarcinoma, refractory to or progressive, following first-line therapy with platinum or fluoropyrimidine chemotherapy. It is estimated that there were approximately 21,600 new cases and 10,990 deaths from gastric cancer in the United States in 2013. The biology of gastric cancer appears to be different in different parts of the globe. Following progression after first line treatment for metastatic disease, the median survival is approximately 3 months. CYRAMZA® is a human IgG1 monoclonal antibody that inhibits VEGF-receptor 2, unlike AVASTIN® (Bevacizumab) which inhibits VEGF-A.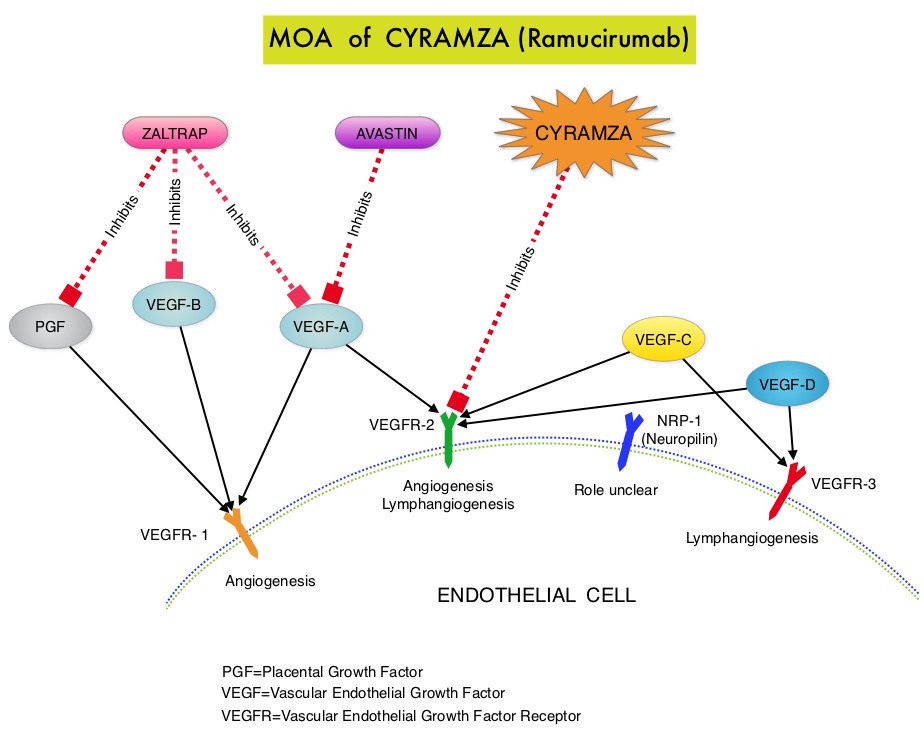 The RAINBOW study is an international, placebo-controlled, double-blind, phase III trial in which 665 patients with metastatic gastroesophageal junction or gastric adenocarcinoma, who had disease progression on or within 4 months after first-line platinum and fluoropyrimidine-based combination therapy, were included. Patients were randomly assigned to receive TAXOL® (Paclitaxel) 80 mg/m2 given on D1, 8, 15 along with Placebo (N=335) or the same dose and schedule of TAXOL® given along with CYRAMZA® at 8 mg/kg IV every 2 weeks (N=330), of a 28 day cycle. Treatment was continued until disease progression or unacceptable toxicities were noted. The primary endpoint was Overall Survival (OS). Secondary endpoints included Progression Free Survival (PFS), Objective Response Rate (ORR) and Time To Progression (TTP). The median OS for the combination of CYRAMZA® and TAXOL® was 9.6 months compared to 7.4 months for Placebo and TAXOL® (HR=0.81; P=0.017), resulting in a 19% reduction in the risk of death with the CYRAMZA® and TAXOL® combination. The secondary endpoints favored the CYRAMZA® and TAXOL® combination as well. The median PFS was 4.4 months and 2.9 months (HR=0.64; P<0.001), ORR was 28% and 16% (P<0.0001) and median TTP was 5.5 months and 3 months with the CYRAMZA® and TAXOL® combination vs Placebo and TAXOL® combination respectively. As one would expect, treatment related adverse events were seen more frequently in the CYRAMZA® and TAXOL® combination group. Significant were neutropenia, hypertension, fatigue and asthenia, diarrhea and epistaxis. The incidence of febrile neutropenia in the two treatment groups was however comparable (3.1% vs 2.4%). The authors concluded that the combination of CYRAMZA® and TAXOL® combination significantly improved both Progression Free and Overall Survival and also resulted in significantly improved disease control rates, in patients with metastatic gastroesophageal junction or gastric adenocarcinoma. Wilke H, Van Cutsem E, Oh SC, et al. J Clin Oncol 32, 2014 (suppl 3; abstr LBA7)
The RAINBOW study is an international, placebo-controlled, double-blind, phase III trial in which 665 patients with metastatic gastroesophageal junction or gastric adenocarcinoma, who had disease progression on or within 4 months after first-line platinum and fluoropyrimidine-based combination therapy, were included. Patients were randomly assigned to receive TAXOL® (Paclitaxel) 80 mg/m2 given on D1, 8, 15 along with Placebo (N=335) or the same dose and schedule of TAXOL® given along with CYRAMZA® at 8 mg/kg IV every 2 weeks (N=330), of a 28 day cycle. Treatment was continued until disease progression or unacceptable toxicities were noted. The primary endpoint was Overall Survival (OS). Secondary endpoints included Progression Free Survival (PFS), Objective Response Rate (ORR) and Time To Progression (TTP). The median OS for the combination of CYRAMZA® and TAXOL® was 9.6 months compared to 7.4 months for Placebo and TAXOL® (HR=0.81; P=0.017), resulting in a 19% reduction in the risk of death with the CYRAMZA® and TAXOL® combination. The secondary endpoints favored the CYRAMZA® and TAXOL® combination as well. The median PFS was 4.4 months and 2.9 months (HR=0.64; P<0.001), ORR was 28% and 16% (P<0.0001) and median TTP was 5.5 months and 3 months with the CYRAMZA® and TAXOL® combination vs Placebo and TAXOL® combination respectively. As one would expect, treatment related adverse events were seen more frequently in the CYRAMZA® and TAXOL® combination group. Significant were neutropenia, hypertension, fatigue and asthenia, diarrhea and epistaxis. The incidence of febrile neutropenia in the two treatment groups was however comparable (3.1% vs 2.4%). The authors concluded that the combination of CYRAMZA® and TAXOL® combination significantly improved both Progression Free and Overall Survival and also resulted in significantly improved disease control rates, in patients with metastatic gastroesophageal junction or gastric adenocarcinoma. Wilke H, Van Cutsem E, Oh SC, et al. J Clin Oncol 32, 2014 (suppl 3; abstr LBA7)

