SUMMARY: Cancer mortality rates in the United States have declined 20% from their peak of 215 per 100,000 in 1991 to 172 per 100,000 in 2010. Part II of this Annual Report on Progress Against Cancer explores, ADVANCES IN TREATMENT, ADVANCES IN TUMOR BIOLOGY AND ADVANCES IN PATIENT CARE. Clinical study details for several of these studies can be accessed at www.oncoprescribe.com
ADVANCES IN TREATMENT
COMBINATION THERAPY
Chemotherapy and Radiotherapy significantly improves Survival for Patients with Low-Grade Glioma – Radiotherapy has been the standard first-line treatment for patients with low-grade glioma. In a study involving 251 patients with gliomas which included grade 2 Astrocytoma, Oligoastrocytoma, or Oligodendroglioma, the addition of chemotherapy (PCV regimen – (Procarbazine, Lomustine, and Vincristine) to radiation extended median survival by 5.5 years (13.3 vs 7.8 years, P=0.03; HR=0.59) and also resulted in a longer median Progression Free survival (10.4 vs 4 years, P=0.002; HR=0.50), when compared with radiotherapy alone.
First-Line Chemotherapy Added to Hormone Therapy Improves Survival for Men With Advanced Prostate Cancer – Androgen Deprivation Therapy (ADT) has been the cornerstone of treatment of advanced prostate cancer and is the first treatment intervention for hormone sensitive prostate cancer. Chemotherapy is usually considered for patients who progress on hormone therapy. In a pivotal phase III study which included 790 men with metastatic, hormone-sensitive prostate cancer, the addition of TAXOTERE® (Docetaxel) chemotherapy to ADT improved median Overall Survival by 10 months from 42.3 months in the ADT alone group to 52.7 months in the ADT plus TAXOTERE® group (HR=0.63; P<0.0006). The median time to clinical progression was 19.8 months in the ADT alone group vs 32.7 months in the ADT plus TAXOTERE® group (P < 0.0001).
TARGETED THERAPY
Overcoming Resistance to EGFR Targeted Agents in Lung Cancer– TARCEVA® (Erlotinib) and GILOTRIF® (Afatinib) are recommended as first-line treatments for patients harboring Epidermal Growth Factor Receptor (EGFR) gene mutations, which are detected in 15% of Caucasian and 40% of Asian patients with NSCLC. An additional EGFR mutation (T790M) is responsible for resistance to EGFR-targeted therapy and may be detected in about 50% of those harboring EGFR mutations in Non Small Cell Lung Cancer (NSCLC). AZD9291 and CO-1686 are two new agents which have demonstrated a 50-60% response rate in patients with T790M mutation.
New second-line treatment options for ALK-positive NSCLC – XALKORI® (Crizotinib) significantly improves PFS and Response Rates in patients with ALK-positive NSCLC. Approximately one third of these patients are resistant to XALKORI® and this has been attributed to acquired mutation within the ALK tyrosine kinase domain, amplification of the ALK fusion gene, subtherapeutic inhibition of ALK tyrosine kinase or activation of other pathways that can cause abnormal cell proliferation. ZYKADIA® (Ceritinib) resulted in an overall Response Rate (RR) of 58% and median PFS of 7 months in this patient population, with responses seen in untreated CNS lesions as well.
FDA Approves First Treatment for Chemotherapy-Resistant, Advanced Stomach Cancer – CYRAMZA® (Ramucirumab) an angiogenesis inhibitor was approved for the treatment of advanced stomach cancer or gastroesophageal junction adenocarcinoma that progressed during or after chemotherapy. The approval was based on a phase III trial involving 355 patients in which patients treated with CYRAMZA® (Ramucirumab) had longer survival (5.2 vs 3.8 months, HR = 0.78, P=0.047), when compared to placebo.
Lenvatinib: A New Option for a Difficult-to-Treat Thyroid Cancer – LENVIMA® (Lenvatinib) a Tyrosine Kinase Inhibitor, was approved for the treatment of patients with locally recurrent or metastatic, progressive, RadioActive Iodine (RAI)-refractory Differentiated Thyroid Cancer (DTC). In a phase III study involving 392 patients with advanced RAI-refractory Differentiated Thyroid Cancer (DTC), the median Progression Free Survival was 18.3 months in the LENVIMA® group and 3.6 months in the placebo group (HR= 0.21; P<0.001). The objective response rate with LENVIMA® was 64.8% versus 1.5% with placebo (P<0.001). Another targeted drug, NEXAVAR® (Sorafenib), was approved for the same patient population in 2013.
IMMUNOTHERAPY
Both antibody and cell-based immunotherapy approaches have taken center stage in cancer immunotherapy. Check point inhibitors such as anti CTLA-4. Anti PD-1 and anti PDL1 antibodies unleash the immune system allowing the T cells to attack the malignant cells.
Adjuvant Immunotherapy For Early Stage Melanoma – YERVOY® (Ipilimumab), an anti CTLA-4 antibody decreased the relative risk of recurrence by 25% (HR=0.75; P=0.0013), when compared with placebo, in patients with high risk, completely resected, stage III melanoma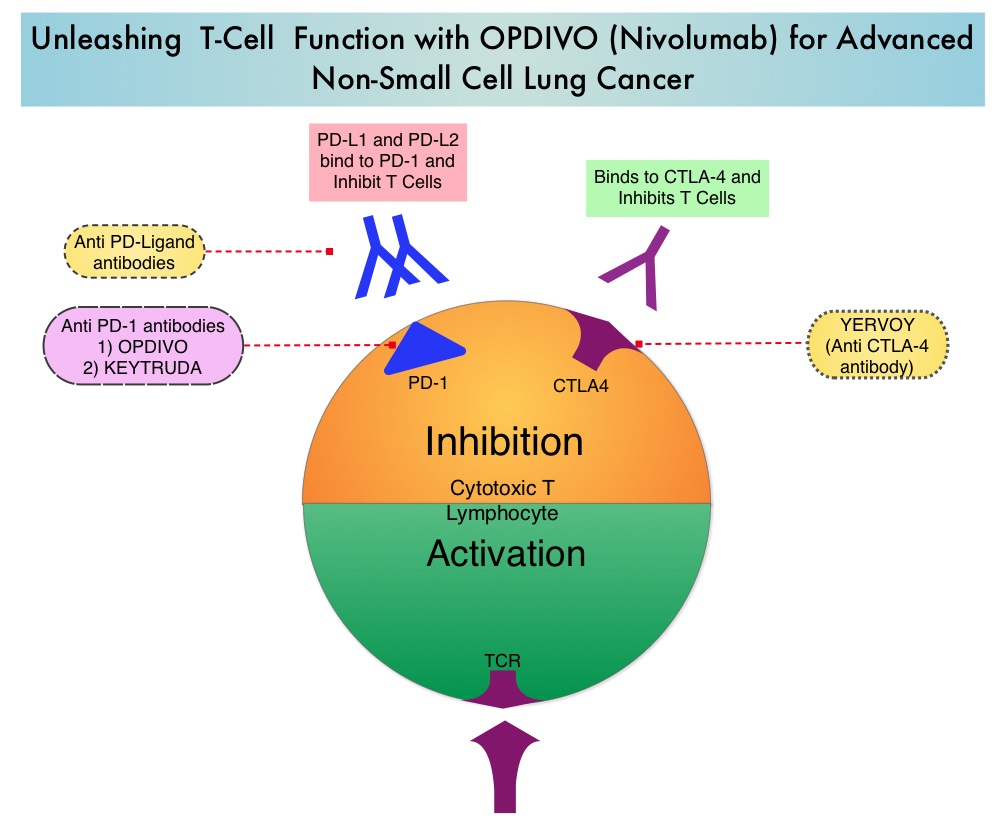
Immunotherapy in Lung Cancer – OPDIVO® (Nivolumab), a PD-1 targeted monoclonal antibody improved overall survival by 41% (HR=0.59; P=0.00025) when compared to TAXOTERE® (Docetaxel) in a randomized phase III trial in patients with metastatic squamous NSCLC, who had experienced disease progression during or after one prior platinum-based chemotherapy regimen. KEYTRUDA® (Pembrolizumab) another PD-1 antibody demonstrated superior responses in patients with lung cancer when the tumors were PD-L1 positive versus PD-L1 negative (23% vs 9%) suggesting that PD-L1 may be a predictor of response to PD-1 and PD-L1 therapies.
Tumor Directed Chimeric Antigen Receptor (CAR) T-Cell Therapy – In this type of immunotherapy, T cells are collected from the patient’s own blood and are genetically engineered to produce special receptors on their surface called chimeric antigen receptors (CAR’s). These cytotoxic T cells with these chimeric antigen receptors on their surface are now able to recognize a specific antigen on tumor cells. These engineered CAR T-cells (CTL019) which are grown in vitro, are then infused into the patient and they in turn proliferate in the patient’s body, recognize and kill cancer cells expressing that specific antigen. In a small study of patients with relapsed or refractory ALL, treatment with autologous Chimeric Antigen Receptor (CAR) T-cells (CTL019 T-cells) resulted in a 90% remission rate with sustained remissions for up to 2 years and overall survival of 78%.
ADVANCES IN TUMOR BIOLOGY
Genomic Profile Based Therapy – In a recent landmark study, analysis of molecular data from approximately 3,500 patients with 12 different forms of cancer, lead to the identification of 11 major molecular subtypes. It was noted that most malignancies that originated from the same tissue or organ had similar genomic profiles. Treatment in the foreseeable future may be based on genomic profile rather than site of origin of the malignancy.
Blood Test Predicts Resistance to Prostate Cancer Therapy – In patients with metastatic CRPC, the presence of Androgen Receptor Variant AR-V7 rather than a normal androgen receptor, in the circulating tumor cells (CTCs) before, during, and after treatment with either XTANDI® (Enzalutamide) or ZYTIGA® (Abiraterone), conferred resistance to these agents. The Androgen Receptor Variant AR-V7 was detected in roughly 40% of patients treated with XTANDI® and 20% of those treated with ZYTIGA®.
Gut Bacteria and Response to Therapy – Two early studies have demonstrated that Intestinal bacteria may be beneficial in priming and mobilizing immune cells to attack tumors. Further, certain chemotherapeutic agents such as CYTOXAN® (Cyclophosphamide) and ELOXATIN® (Oxaliplatin) were less effective when intestinal bacteria were eradicated with antibiotics.
ADVANCES IN PATIENT CARE
Early Initiation of Palliative Care Improves Patient Well Being – In a study of close to 500 patients with advanced cancer early palliative care improved, quality of life at the end of life, spiritual well-being, symptom severity, and satisfaction with care at 4 months after diagnosis.
Pregnancy After Breast Cancer Treatment – Premature Ovarian Failure (POF) is a common unintended consequence of chemotherapy in premenopausal women. Besides loss of fertility, which can influence treatment decisions in young women, ovarian failure can lead to menopausal symptoms, sexual dysfunction and loss of bone density. Two studies reported a promising new way of preserve fertility. In the POEMS (Prevention of Early Menopause Study) phase III trial, the addition of ZOLADEX® (Goserelin) to chemotherapy decreased the POF to 8% compared to 22% with chemotherapy alone (P=0.04). More women in the ZOLADEX® group achieved at least one pregnancy (21%) compared to 11% in the chemotherapy alone group (P=0.03). In a different study, similar findings were noted with the addition of another hormonal agent, TRELSTAR® (Triptorelin) to chemotherapy.
Masters GA, Krilov L, Bailey HH , et al. Published online before print January 20, 2015, doi: 10.1200/JCO.2014.59.9746
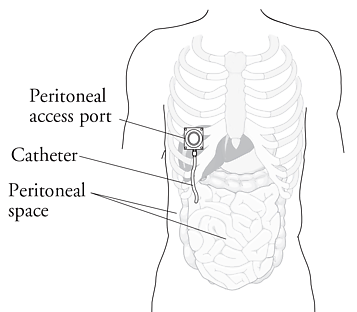 IP chemotherapy for ovarian cancer facilitates the exposure of tumors in the peritoneal cavity to 10-20 fold greater concentration of Cisplatin and Carboplatin and 1000 fold greater concentration of Paclitaxel, compared to IV administration, thus allowing continuous and prolonged exposure of the tumor to high drug concentrations, without systemic toxicities. Even though three Intergroup Phase III trials demonstrated the superiority of IP therapy over IV therapy, it has not been widely accepted in the US and abroad. Barriers to IP therapy have included inconvenience, IP catheter related complications, higher toxicities, lack of knowledge regarding patient selection for IP therapy as well as minimum number of cycles of IP therapy to administer and uncertain long term benefit.
IP chemotherapy for ovarian cancer facilitates the exposure of tumors in the peritoneal cavity to 10-20 fold greater concentration of Cisplatin and Carboplatin and 1000 fold greater concentration of Paclitaxel, compared to IV administration, thus allowing continuous and prolonged exposure of the tumor to high drug concentrations, without systemic toxicities. Even though three Intergroup Phase III trials demonstrated the superiority of IP therapy over IV therapy, it has not been widely accepted in the US and abroad. Barriers to IP therapy have included inconvenience, IP catheter related complications, higher toxicities, lack of knowledge regarding patient selection for IP therapy as well as minimum number of cycles of IP therapy to administer and uncertain long term benefit.

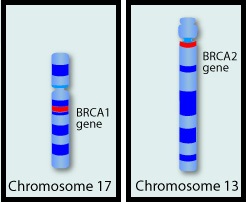 They control cell growth by repairing DNA damage and thus prevent tumor development. Mutations in these genes predispose an individual to develop malignant tumors. The presence of BRCA1 and BRCA2 mutations can significantly increase the lifetime risk for developing Breast and Ovarian cancer, as high as 85% and 40% respectively. While the association of BRCA1 and BRCA2 mutations with Breast and Ovarian cancer risks is well-established, the potential association of these mutations with other cancers has remained unclear. This study was conducted to evaluate the incidence of cancers other than Breast and Ovarian cancer, in known BRCA1 and BRCA2 mutation carriers. The study population included 1072 patients who had received genetic counseling at the UT MD Anderson Cancer Center between 1997 and 2013 and had a confirmed BRCA1 (N=613) or BRCA2 (N=459) mutation. The authors then compared the cancer incidence of the study population with the United States Cancer Statistics. The expected and observed numbers of cancer cases were calculated at 5 year intervals to take into consideration different age-related incidence rates. Standardized Incidence Ratios (SIRs) for each cancer type was then calculated for the entire sample and for BRCA1 mutation carriers and BRCA2 mutation carriers separately. {Standardized Incidence Ratio (SIR) is used to determine if the occurrence of cancer in a relatively small population is high or low. A SIR of 1 would indicate no increase or decrease, SIR of 1.5 indicates an excess of 50%, SIR of 2 indicates an excess of 100%}. Among the study population, 1177 cancers comprising 30 different cancer types were identified. There was no significant increase in cancers other than Breast and Ovarian cancer in individuals with BRCA1 mutation, but there was a trend of increasing incidence of Melanoma in BRCA1 mutation carriers, compared to general population. Individuals with BRCA2 mutation however had significantly higher incidence of Pancreatic cancer in both men and women (SIR= 21.7; P < 0.001), Prostate cancer in men (SIR= 4.9; P = 0.002) and a trend of increasing incidence of Cervical cancer, compared to general population. The authors concluded that their findings support the NCCN practice guidelines for this patient population which includes screening for male Breast cancer, Prostate cancer and Melanoma, acknowledging that specific screening guidelines for Pancreatic cancer do not exist. Mersch J, Jackson MA, Park M, et al. Cancer 2015; 121:269-275
They control cell growth by repairing DNA damage and thus prevent tumor development. Mutations in these genes predispose an individual to develop malignant tumors. The presence of BRCA1 and BRCA2 mutations can significantly increase the lifetime risk for developing Breast and Ovarian cancer, as high as 85% and 40% respectively. While the association of BRCA1 and BRCA2 mutations with Breast and Ovarian cancer risks is well-established, the potential association of these mutations with other cancers has remained unclear. This study was conducted to evaluate the incidence of cancers other than Breast and Ovarian cancer, in known BRCA1 and BRCA2 mutation carriers. The study population included 1072 patients who had received genetic counseling at the UT MD Anderson Cancer Center between 1997 and 2013 and had a confirmed BRCA1 (N=613) or BRCA2 (N=459) mutation. The authors then compared the cancer incidence of the study population with the United States Cancer Statistics. The expected and observed numbers of cancer cases were calculated at 5 year intervals to take into consideration different age-related incidence rates. Standardized Incidence Ratios (SIRs) for each cancer type was then calculated for the entire sample and for BRCA1 mutation carriers and BRCA2 mutation carriers separately. {Standardized Incidence Ratio (SIR) is used to determine if the occurrence of cancer in a relatively small population is high or low. A SIR of 1 would indicate no increase or decrease, SIR of 1.5 indicates an excess of 50%, SIR of 2 indicates an excess of 100%}. Among the study population, 1177 cancers comprising 30 different cancer types were identified. There was no significant increase in cancers other than Breast and Ovarian cancer in individuals with BRCA1 mutation, but there was a trend of increasing incidence of Melanoma in BRCA1 mutation carriers, compared to general population. Individuals with BRCA2 mutation however had significantly higher incidence of Pancreatic cancer in both men and women (SIR= 21.7; P < 0.001), Prostate cancer in men (SIR= 4.9; P = 0.002) and a trend of increasing incidence of Cervical cancer, compared to general population. The authors concluded that their findings support the NCCN practice guidelines for this patient population which includes screening for male Breast cancer, Prostate cancer and Melanoma, acknowledging that specific screening guidelines for Pancreatic cancer do not exist. Mersch J, Jackson MA, Park M, et al. Cancer 2015; 121:269-275
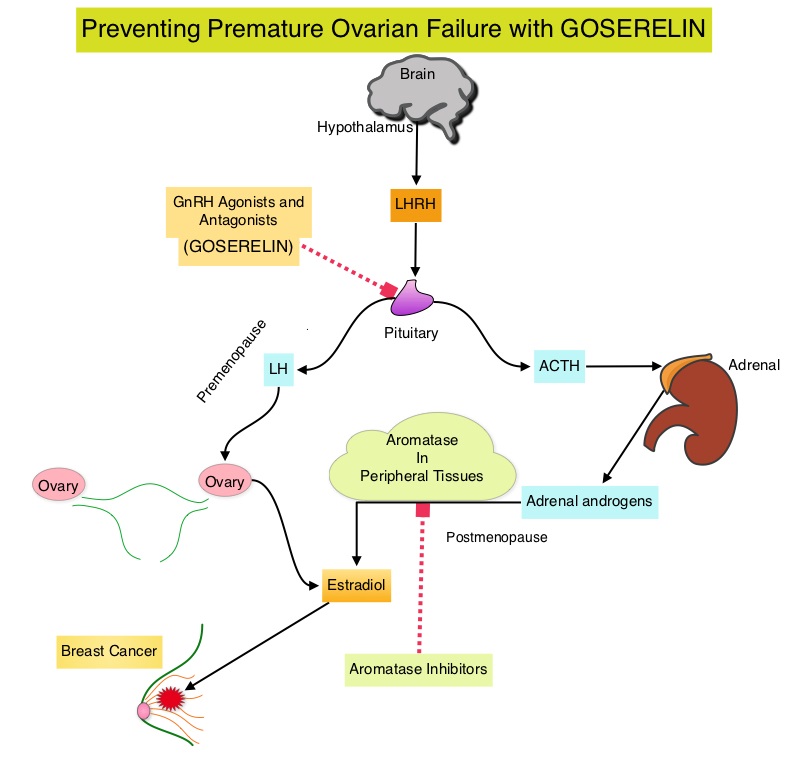 Premature Ovarian Failure (POF) is a common unintended consequence of chemotherapy in premenopausal women. Besides of loss of fertility, which can influence treatment decisions in young women, ovarian failure can lead to menopausal symptoms, sexual dysfunction and loss of bone density. POEMS (Prevention of Early Menopause Study) is a randomized phase III trial designed to evaluate whether the addition of LHRH (Luteinizing Hormone-Releasing Hormone) analog Goserelin (ZOLADEX®), which suppresses the production of estrogens, to Cyclophosphamide based chemotherapy, would reduce POF in breast cancer patients, when compared to chemotherapy alone. Premenopausal patients less than 50 years of age, with hormone receptor negative (ER/PR negative ), Stage I-IIIA breast cancer, scheduled to receive chemotherapy, were randomly assigned to receive standard Cyclophosphamide based chemotherapy with or without monthly ZOLADEX® . Patients in the ZOLADEX® group received 3.6 mg SQ starting 1 week prior to the first dose of chemotherapy. The primary endpoint was ovarian failure at two years (defined as amenorrhea for the prior 6 months AND post-menopausal FSH level). Other endpoints included pregnancy and survival rates. The median age of the patients was 38 years and median follow up was 4.1 years. Of the 218 evaluable patients, 135 premenopausal women were evaluable for the primary end point. POF rates were 22% in the chemotherapy alone group and 8% in the ZOLADEX® group (P=0.04). When the definition of POF was more liberal to include EITHER amenorrhea or elevated FSH but not both, POF rates were 45% in the chemotherapy alone group and 20% in the ZOLADEX® group (P=0.006). Among the 218 evaluable patients, more women in the ZOLADEX® group achieved at least one pregnancy (21%) compared to 11% in the chemotherapy alone group (P=0.03). Secondary outcomes also favored the ZOLADEX® group with a Disease free Survival (DFS) rate of 78% in the chemotherapy alone group compared with 89% in the ZOLADEX® group (P=0.04) and Overall Survival (OS) rate of 82% in the chemotherapy alone group compared with 92% in the ZOLADEX® group (P=0.05). The authors concluded that the addition of ZOLADEX® to chemotherapy improved fertility prospects with a lower incidence of Premature Ovarian Failure and more pregnancies. Further, the improved Disease Free Survival and Overall Survival is an important additional perk and prevention of Premature Ovarian Failure with ZOLADEX® may be a consideration not only in premenopausal patients with hormone receptor positive breast cancer but also in other malignancies such as lymphomas, when treated with similar chemotherapeutic agents. Goserelin for Ovarian Protection during Breast-Cancer Adjuvant Chemotherapy. Moore HC, Unger JM, Phillips K, et al. N Engl J Med 2015; 372:923-932
Premature Ovarian Failure (POF) is a common unintended consequence of chemotherapy in premenopausal women. Besides of loss of fertility, which can influence treatment decisions in young women, ovarian failure can lead to menopausal symptoms, sexual dysfunction and loss of bone density. POEMS (Prevention of Early Menopause Study) is a randomized phase III trial designed to evaluate whether the addition of LHRH (Luteinizing Hormone-Releasing Hormone) analog Goserelin (ZOLADEX®), which suppresses the production of estrogens, to Cyclophosphamide based chemotherapy, would reduce POF in breast cancer patients, when compared to chemotherapy alone. Premenopausal patients less than 50 years of age, with hormone receptor negative (ER/PR negative ), Stage I-IIIA breast cancer, scheduled to receive chemotherapy, were randomly assigned to receive standard Cyclophosphamide based chemotherapy with or without monthly ZOLADEX® . Patients in the ZOLADEX® group received 3.6 mg SQ starting 1 week prior to the first dose of chemotherapy. The primary endpoint was ovarian failure at two years (defined as amenorrhea for the prior 6 months AND post-menopausal FSH level). Other endpoints included pregnancy and survival rates. The median age of the patients was 38 years and median follow up was 4.1 years. Of the 218 evaluable patients, 135 premenopausal women were evaluable for the primary end point. POF rates were 22% in the chemotherapy alone group and 8% in the ZOLADEX® group (P=0.04). When the definition of POF was more liberal to include EITHER amenorrhea or elevated FSH but not both, POF rates were 45% in the chemotherapy alone group and 20% in the ZOLADEX® group (P=0.006). Among the 218 evaluable patients, more women in the ZOLADEX® group achieved at least one pregnancy (21%) compared to 11% in the chemotherapy alone group (P=0.03). Secondary outcomes also favored the ZOLADEX® group with a Disease free Survival (DFS) rate of 78% in the chemotherapy alone group compared with 89% in the ZOLADEX® group (P=0.04) and Overall Survival (OS) rate of 82% in the chemotherapy alone group compared with 92% in the ZOLADEX® group (P=0.05). The authors concluded that the addition of ZOLADEX® to chemotherapy improved fertility prospects with a lower incidence of Premature Ovarian Failure and more pregnancies. Further, the improved Disease Free Survival and Overall Survival is an important additional perk and prevention of Premature Ovarian Failure with ZOLADEX® may be a consideration not only in premenopausal patients with hormone receptor positive breast cancer but also in other malignancies such as lymphomas, when treated with similar chemotherapeutic agents. Goserelin for Ovarian Protection during Breast-Cancer Adjuvant Chemotherapy. Moore HC, Unger JM, Phillips K, et al. N Engl J Med 2015; 372:923-932
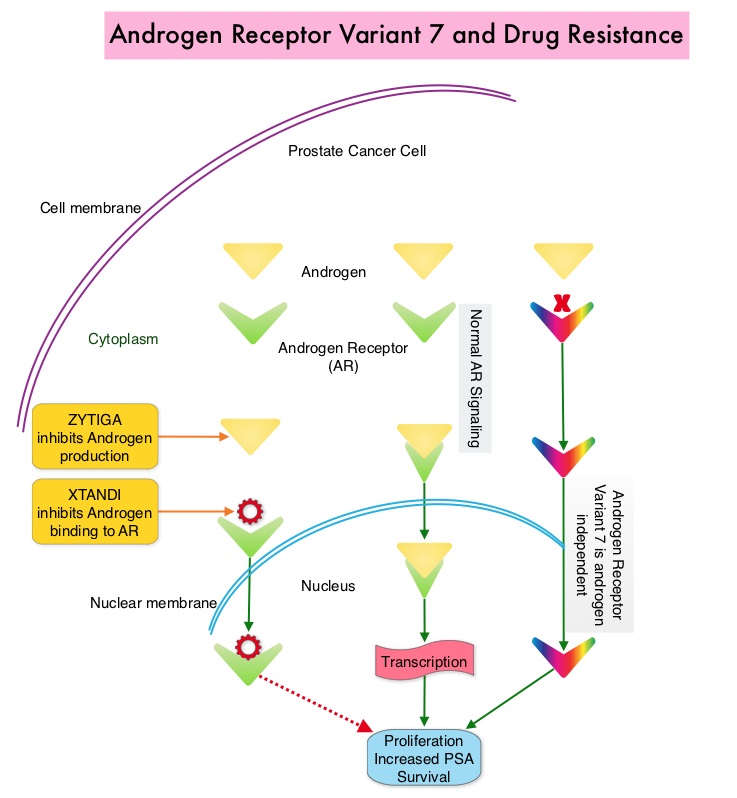 Both these agents have been shown to improve survival in metastatic CRPC. ZYTIGA® inhibits CYP 17A1 enzyme and depletes adrenal and intratumoral androgens, thereby impairing AR signaling. XTANDI® competes with Testosterone and Dihydrotestosterone and avidly binds to the Androgen Receptor, thereby inhibiting AR signaling and in addition inhibits translocation of the AR into the nucleus and thus inhibits the transcriptional activities of the AR. About 20-40% of the patients do not respond to these newer agents and even those who respond will invariably develop resistance to these drugs. This again has been attributed to persistent AR signaling by variant forms of Androgen Receptor, generated through somatic mutation or aberrant RNA splicing. Androgen Receptor Variant AR-V7 can be detected in the CTCs (Circulating Tumor Cells). AR-V7 does not have the domain to bind androgens and may be associated with resistance to XTANDI®. Further AR-V7 is constitutively active and can independently activate transcription factors and therefore is not effected by androgen depleting agents including ZYTIGA®. With this background, the authors hypothesized that detection of Androgen Receptor variant AR-V7 in circulating tumor cells from men with metastatic prostate cancer would be associated with resistance to both ZYTIGA® and XTANDI®. In a previously published prospective study, data involving 62 patients showed that detection of AR-V7 in Circulating Tumor Cells (CTCs) in men with mCRPC was indeed associated with primary resistance to both ZYTIGA® and XTANDI®. AR-V7–positive patients had inferior overall survival with both XTANDI® (HR = 6.9; P =0.002) and ZYTIGA® (HR = 12.7; P =0.006). AR-V7 was therefore shown to have a prognostic value for outcomes in mCRPC with ZYTIGA® and XTANDI®. In this present publication, the authors studied to determine if AR-V7-positive patients would retain sensitivity to Taxane chemotherapy. The researchers in this small prospective study enrolled 37 patients with metastatic CRPC who were starting Taxane chemotherapy with Cabazitaxel (JEVTANA®) or Docetaxel (TAXOTERE®). Presence or lack of AR-V7 in circulating tumor cells (CTCs), was determined by the qRT-PCR assay. Of the enrolled patients, 46% had detectable AR-V7 in CTCs. The primary endpoint was associations between AR-V7 status and PSA response rates and secondary endpoints included Progression Free Survival (PFS). They noted that the PSA responses were achieved in both AR-V7- positive and AR-V7-negative men and the difference was non-significant (41% versus 65%, P=0.19). Likewise there was no significant difference in the median PFS in AR-V7-positive and AR-V7-negative men (5.1 versus 6.9 months (HR= 2.65; P=0.11). The researchers then combined the data from their previously published study with 62 patients and they noted that, in AR-V7-positive men, PSA responses were higher in Taxane treated versus ZYTIGA®/XTANDI® treated men (41% versus 0%, P<0.001) and PFS were longer in the Taxane treated men as well (HR for PFS = 0.21, P=0.003). The outcomes however did not differ by treatment type in AR-V7-negative men and were comparable. The authors concluded that detection of AR-V7 in CTCs from men with mCRPC is not associated with primary resistance to Taxane chemotherapy, and such patients may retain sensitivity to Taxanes. In AR-V7-positive men however, Taxanes appear to be more efficacious than ZYTIGA® or XTANDI®. AR-V7 once available commercially, may become a biomarker for treatment selection, in metastatic Castrate Resistant Prostate Cancer. AR splice variant 7 (AR-V7) and response to taxanes in men with metastatic castration-resistant prostate cancer (mCRPC). Antonarakis ES, Lu C, Chen Y, et al. J Clin Oncol 33, 2015 (suppl 7; abstr 138)
Both these agents have been shown to improve survival in metastatic CRPC. ZYTIGA® inhibits CYP 17A1 enzyme and depletes adrenal and intratumoral androgens, thereby impairing AR signaling. XTANDI® competes with Testosterone and Dihydrotestosterone and avidly binds to the Androgen Receptor, thereby inhibiting AR signaling and in addition inhibits translocation of the AR into the nucleus and thus inhibits the transcriptional activities of the AR. About 20-40% of the patients do not respond to these newer agents and even those who respond will invariably develop resistance to these drugs. This again has been attributed to persistent AR signaling by variant forms of Androgen Receptor, generated through somatic mutation or aberrant RNA splicing. Androgen Receptor Variant AR-V7 can be detected in the CTCs (Circulating Tumor Cells). AR-V7 does not have the domain to bind androgens and may be associated with resistance to XTANDI®. Further AR-V7 is constitutively active and can independently activate transcription factors and therefore is not effected by androgen depleting agents including ZYTIGA®. With this background, the authors hypothesized that detection of Androgen Receptor variant AR-V7 in circulating tumor cells from men with metastatic prostate cancer would be associated with resistance to both ZYTIGA® and XTANDI®. In a previously published prospective study, data involving 62 patients showed that detection of AR-V7 in Circulating Tumor Cells (CTCs) in men with mCRPC was indeed associated with primary resistance to both ZYTIGA® and XTANDI®. AR-V7–positive patients had inferior overall survival with both XTANDI® (HR = 6.9; P =0.002) and ZYTIGA® (HR = 12.7; P =0.006). AR-V7 was therefore shown to have a prognostic value for outcomes in mCRPC with ZYTIGA® and XTANDI®. In this present publication, the authors studied to determine if AR-V7-positive patients would retain sensitivity to Taxane chemotherapy. The researchers in this small prospective study enrolled 37 patients with metastatic CRPC who were starting Taxane chemotherapy with Cabazitaxel (JEVTANA®) or Docetaxel (TAXOTERE®). Presence or lack of AR-V7 in circulating tumor cells (CTCs), was determined by the qRT-PCR assay. Of the enrolled patients, 46% had detectable AR-V7 in CTCs. The primary endpoint was associations between AR-V7 status and PSA response rates and secondary endpoints included Progression Free Survival (PFS). They noted that the PSA responses were achieved in both AR-V7- positive and AR-V7-negative men and the difference was non-significant (41% versus 65%, P=0.19). Likewise there was no significant difference in the median PFS in AR-V7-positive and AR-V7-negative men (5.1 versus 6.9 months (HR= 2.65; P=0.11). The researchers then combined the data from their previously published study with 62 patients and they noted that, in AR-V7-positive men, PSA responses were higher in Taxane treated versus ZYTIGA®/XTANDI® treated men (41% versus 0%, P<0.001) and PFS were longer in the Taxane treated men as well (HR for PFS = 0.21, P=0.003). The outcomes however did not differ by treatment type in AR-V7-negative men and were comparable. The authors concluded that detection of AR-V7 in CTCs from men with mCRPC is not associated with primary resistance to Taxane chemotherapy, and such patients may retain sensitivity to Taxanes. In AR-V7-positive men however, Taxanes appear to be more efficacious than ZYTIGA® or XTANDI®. AR-V7 once available commercially, may become a biomarker for treatment selection, in metastatic Castrate Resistant Prostate Cancer. AR splice variant 7 (AR-V7) and response to taxanes in men with metastatic castration-resistant prostate cancer (mCRPC). Antonarakis ES, Lu C, Chen Y, et al. J Clin Oncol 33, 2015 (suppl 7; abstr 138)