SUMMARY: The American Cancer Society estimates that about 71,850 people will be diagnosed with Non-Hodgkin Lymphoma (NHL) in the United States and about 19,800 individuals will die of this disease. Approximately 20% of all NHLs are Follicular Lymphomas (FL). Follicular Lymphoma is the most indolent form and second most common form of all NHLs and they are a heterogeneous group of lymphoproliferative malignancies. Advanced stage Follicular lymphomas are not curable and as such prolonging Progression Free Survival (PFS) and Overall Survival (OS) while maintaining quality of life (QoL), has been the goals of treatment intervention. Asymptomatic patients with FL are generally considered candidates for “watch and wait” approach, whereas those with B symptoms (fever, night sweats, and weight loss), painful lymphadenopathy/splenomegaly, organ compromise and cytopenias are generally considered candidates for therapy. Follicular Lymphoma International Prognostic Index (FLIPI) is of prognostic value and is used to help with treatment choices. The Ann Arbor classification divides FL into four stages. Patients with stages I and II have localized disease and those with stages III and IV have advanced disease. The World Health Organization (WHO) further classified FL based on histology into low grade (grades 1 and 2) and high grade (grade 3a) FLs. Grade 3b FL which demonstrates diffuse areas of involvement is designated as Diffuse Large B-cell Lymphoma (DLBCL) and is treated as such.
The authors in this publication following review of literature from 2000-2014, addressed FOUR important questions
What treatment options should be considered for localized FL?
Recommendation 1: The preferred treatment for localized FL is Radiation Therapy (RT).
Recommendation 2: Given the potential for long term toxicities, lower doses of RT (24 to 30 Gy) in 1.5 to 2Gy fractions and smaller field sizes, is recommended.
Recommendation 3: Combined modality treatments may be considered for patients with localized disease if positive outcomes are proven in randomized studies.
Recommendation 4: Observation alone may be a reasonable alternative if the potential toxicity of RT outweighs the potential benefits or if the patient refuses RT.
How should asymptomatic advanced-stage FL be managed?
Recommendation 1: Initiation of Chemotherapy or Immunotherapy should be based on the identification of symptoms as defined by the GELF or BNLI criteria.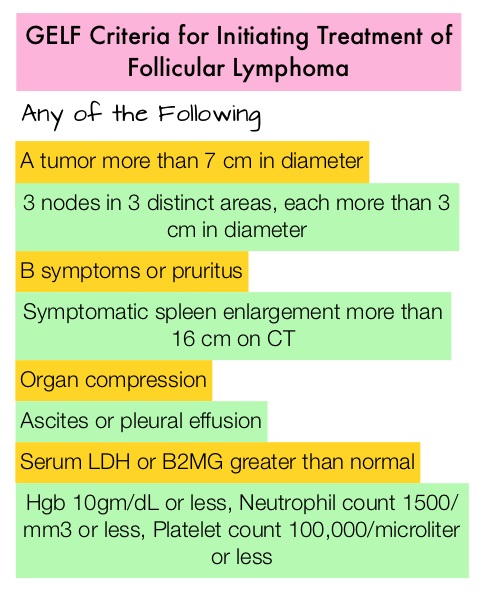
Recommendation 2: Chemoimmunotherapy or Chemotherapy alone as early treatment is not recommended in asymptomatic advanced FL, because of the lack of published randomized studies. Observation alone is therefore recommended in patients who do not meet the criteria for treatment with Chemotherapy or Immunotherapy.
Recommendation 3: If ongoing randomized studies show positive outcomes with reduced risk of relapse, early treatment with RITUXAN® (Rituximab) with or without RITUXAN® maintenance in asymptomatic patients may be considered.
What treatment options should be considered for symptomatic advanced-stage FL?
Recommendation 1: Based on the lower response rate with RITUXAN® monotherapy, Chemoimmunotherapy is preferred over RITUXAN® monotherapy , for the first-line treatment of symptomatic advanced stage FL, except when Chemotherapy is contraindicated.
Recommendation 2: RITUXAN® should be added to chemotherapy in the first line treatment of symptomatic advanced-stage FL given the improved Overall Response Rate (ORR) and Progression Free survival (PFS) demonstrated with the addition of RITUXAN® to a number of Chemotherapy combinations.
Recommendation 3: TREANDA® (Bendamustine) and RITUXAN® (BR) is the preferred Chemoimmunotherapy regimen for the first-line treatment of symptomatic advanced-stage FL given the superior efficacy and favorable tolerability of this regimen compared to R-CHOP, confirmed in 2 randomized trials.
In which patients should additional treatment be considered (ie, maintenance, consolidation, SCT)?
Recommendation 1: Maintenance RITUXAN® is recommended after first-line treatment of FL given the improved response with RITUXAN® maintenance versus observation, demonstrated in 2 randomized trials. The optimal frequency and duration of maintenance RITUXAN® is presently unclear.
Recommendation 2: High Dose Therapy (HDT) followed by Autologous Stem Cell Transplantation (ASCT) is not recommended as part of front-line treatment of FL, given the lack of a survival benefit and the potential toxicity of this approach. There also is no evidence to support the use of Radioimmunotherapy (RIT) after first-line treatment of FL.
A Canadian Evidence-Based Guideline for the First-Line Treatment of Follicular Lymphoma: Joint Consensus of the Lymphoma Canada Scientific Advisory Board. Kuruvilla J, Assouline S, Hodgson D, et al. Clinical Lymphoma Myeloma and Leukemia 2015; 15:59-74

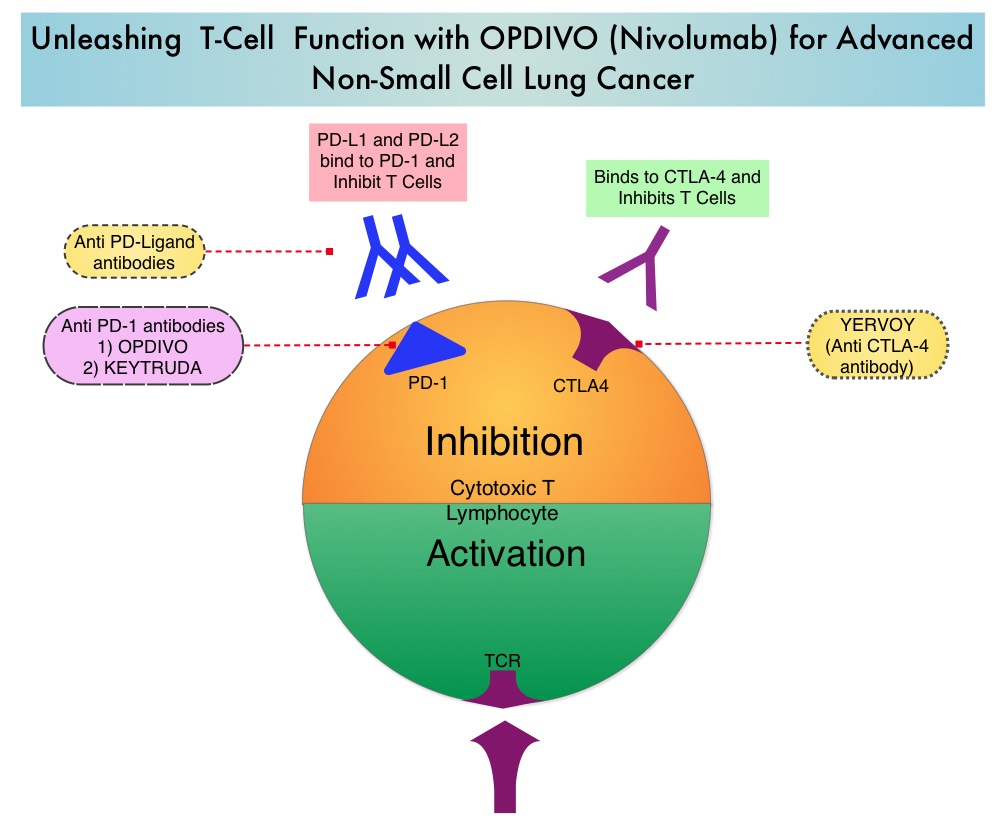 The development a novel immunotherapeutic approaches, with a better understanding of the Immune checkpoints has however changed the treatment paradigm. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent, related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4), also known as CD152, PD-1(Programmed cell Death-1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. The first Immune checkpoint protein to be clinically targeted was CTLA-4. YERVOY® (Ipilimumab), an antibody that blocks Immune checkpoint protein/receptor CTLA- 4, has been shown to prolong overall survival in patients with previously treated, unresectable or metastatic melanoma. OPDIVO® (Nivolumab) is a fully human, immunoglobulin G4 monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the T cells. The approval of OPDIVO® was based on CheckMate-017, an open label, multicenter, multinational randomized phase III trial in which 272 patients with metastatic squamous NSCLC who had experienced disease progression during or after one prior platinum-based chemotherapy regimen were randomized to receive OPDIVO® (Nivolumab) 3 mg/kg IV every 2 weeks (N=135) or TAXOTERE® (Docetaxel) 75 mg/m2 IV every 3 weeks (N=137). The primary endpoint was Overall Survival (OS) and secondary endpoints included Progression Free Survival (PFS) and Objective Response Rate (ORR). This study was stopped early at the protocol pre-specified interim analysis after an independent monitoring panel determined that the primary endpoint of improved Overall Survival (OS) with OPDIVO® had been reached. The median OS was 9.2 months for patients assigned to OPDIVO® and 6 months for those in the TAXOTERE® group (HR=0.59; P=0.00025). This suggested a 41% improvement in the OS with OPDIVO® compared to TAXOTERE®. This FDA approval was further supported by a single arm, multinational, multicenter trial in patients with metastatic squamous NSCLC (N=117) who had progressed after receiving a platinum-based therapy and at least one additional systemic regimen. OPDIVO® in this study, was administered as a single agent at 3mg/kg IV every two weeks until disease progression or treatment discontinuation. The primary endpoint was Objective Response Rate (ORR) and exploratory endpoints were Overall Survival (OS), Progression Free Survival (PFS) and efficacy, based on PD-L1 expression status. With 11 months of minimum follow up, the Objective Response Rate (ORR) was 15% independent of PD-L1 status. The estimated one-year survival rate was 41% and median Overall Survival was 8.2 months. The authors noted that an additional 26% of patients had stable disease for a median duration of 6 months, resulting in a disease control rate (ORR+stable disease) of 41%. The most frequent grade 3-4 adverse events noted in at least 5% of the patients were fatigue, dyspnea and musculoskeletal pain. OPDIVO® will now be a new treatment option, with survival advantage, for patients with advanced relapsed and refractory metastatic squamous NSCLC. Phase II study of nivolumab (Anti-PD-1, BMS-936558, ONO-4538) in patients with advanced, refractory squamous non-small cell lung cancer. Ramalingam SS, Mazieres J, Planchard D, et al. Presented at: 2014 Multidisciplinary Symposium in Thoracic Oncology; Chicago, IL. LBA#3462
The development a novel immunotherapeutic approaches, with a better understanding of the Immune checkpoints has however changed the treatment paradigm. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent, related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4), also known as CD152, PD-1(Programmed cell Death-1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. The first Immune checkpoint protein to be clinically targeted was CTLA-4. YERVOY® (Ipilimumab), an antibody that blocks Immune checkpoint protein/receptor CTLA- 4, has been shown to prolong overall survival in patients with previously treated, unresectable or metastatic melanoma. OPDIVO® (Nivolumab) is a fully human, immunoglobulin G4 monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the T cells. The approval of OPDIVO® was based on CheckMate-017, an open label, multicenter, multinational randomized phase III trial in which 272 patients with metastatic squamous NSCLC who had experienced disease progression during or after one prior platinum-based chemotherapy regimen were randomized to receive OPDIVO® (Nivolumab) 3 mg/kg IV every 2 weeks (N=135) or TAXOTERE® (Docetaxel) 75 mg/m2 IV every 3 weeks (N=137). The primary endpoint was Overall Survival (OS) and secondary endpoints included Progression Free Survival (PFS) and Objective Response Rate (ORR). This study was stopped early at the protocol pre-specified interim analysis after an independent monitoring panel determined that the primary endpoint of improved Overall Survival (OS) with OPDIVO® had been reached. The median OS was 9.2 months for patients assigned to OPDIVO® and 6 months for those in the TAXOTERE® group (HR=0.59; P=0.00025). This suggested a 41% improvement in the OS with OPDIVO® compared to TAXOTERE®. This FDA approval was further supported by a single arm, multinational, multicenter trial in patients with metastatic squamous NSCLC (N=117) who had progressed after receiving a platinum-based therapy and at least one additional systemic regimen. OPDIVO® in this study, was administered as a single agent at 3mg/kg IV every two weeks until disease progression or treatment discontinuation. The primary endpoint was Objective Response Rate (ORR) and exploratory endpoints were Overall Survival (OS), Progression Free Survival (PFS) and efficacy, based on PD-L1 expression status. With 11 months of minimum follow up, the Objective Response Rate (ORR) was 15% independent of PD-L1 status. The estimated one-year survival rate was 41% and median Overall Survival was 8.2 months. The authors noted that an additional 26% of patients had stable disease for a median duration of 6 months, resulting in a disease control rate (ORR+stable disease) of 41%. The most frequent grade 3-4 adverse events noted in at least 5% of the patients were fatigue, dyspnea and musculoskeletal pain. OPDIVO® will now be a new treatment option, with survival advantage, for patients with advanced relapsed and refractory metastatic squamous NSCLC. Phase II study of nivolumab (Anti-PD-1, BMS-936558, ONO-4538) in patients with advanced, refractory squamous non-small cell lung cancer. Ramalingam SS, Mazieres J, Planchard D, et al. Presented at: 2014 Multidisciplinary Symposium in Thoracic Oncology; Chicago, IL. LBA#3462
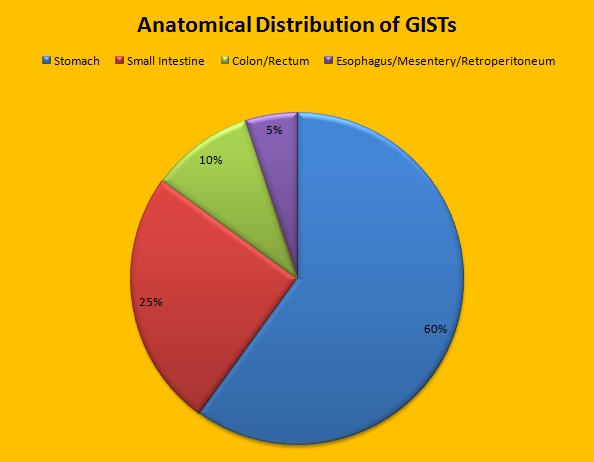 Approximately 60% of the primary GISTs originate in the stomach, 25% in the small intestine, 10% in the colon and rectum and the rest at other sites such as the esophagus, mesentery and retroperitoneum. GISTs were misclassified as leiomyomas or leiomyosarcomas until 1998, when the discovery of mutations in the c-KIT oncogene, lead to a better understanding of these tumors. C-KIT oncogene encodes the transmembrane KIT receptor tyrosine kinase. Approximately 85% of the GISTs have activating (gain of function) KIT mutations and are positive for the CD117 antigen, an epitope of KIT receptor tyrosine kinase. Positive CD117 by ImmunoHistoChemistry (IHC) is however not specific for GIST, as weak reactivity occurs with other mesenchymal tumors. IHC staining for protein kinase C theta and DOG1 are helpful in distinguishing GIST from other mesenchymal tumors, particularly those that are KIT-negative. DOG1 (Discovered On GIST 1) is a protein of unknown function that is expressed strongly in GISTs and is rarely expressed in other mesenchymal neoplasms. KIT mutations in GISTs occur in Exon 9 (10%), Exon 11 (67%), Exon 13 (1%) and Exon 17 (1%). About 5% of the GISTs have activating mutation in the Platelet-Derived Growth Factor Receptor alpha (PDGFRA) gene which encodes for another tyrosine kinase receptor. Approximately 10% to 20% of GISTs have no KIT or PDGFRA mutations and are referred as wild-type GISTs. Mutational status is important as they may predict response to GLEEVEC® and also have prognostic significance. Patients with KIT exon 11 mutations are most sensitive and have a much higher response to GLEEVEC® whereas those with KIT exon 9 mutation or wild-type c-KIT may require a higher dose of GLEEVEC® (800 mg daily dose). KIT exon 11 mutations affecting the codons 557 and 558 is an independent adverse prognostic factor and associated with higher incidence of metastases whereas GISTs with KIT exon 9 mutations usually arise in the small bowel and are associated with frequent recurrences. GISTs with PDGFRA mutations in general have low mitotic count and low malignant potential and those with PDGFRA exon 18 mutation have favorable survival outcomes and located in the stomach. They are however resistant to GLEEVEC®. Although mutated KIT and PDGFRA have been identified as important driver mutations for GIST oncogenesis, the clinical significance of their single mutations has been unclear. To address this, the authors in this study identified 3067 patients with GIST from databases who had macroscopically complete tumor excision and had no detectable metastases at the time of diagnosis. Information on mutation analysis was available on 1505 tumors. The researchers then analyzed associations between KIT and PDGFRA mutations and Recurrence Free Survival (RFS) in this patient population treated with surgery alone.
Approximately 60% of the primary GISTs originate in the stomach, 25% in the small intestine, 10% in the colon and rectum and the rest at other sites such as the esophagus, mesentery and retroperitoneum. GISTs were misclassified as leiomyomas or leiomyosarcomas until 1998, when the discovery of mutations in the c-KIT oncogene, lead to a better understanding of these tumors. C-KIT oncogene encodes the transmembrane KIT receptor tyrosine kinase. Approximately 85% of the GISTs have activating (gain of function) KIT mutations and are positive for the CD117 antigen, an epitope of KIT receptor tyrosine kinase. Positive CD117 by ImmunoHistoChemistry (IHC) is however not specific for GIST, as weak reactivity occurs with other mesenchymal tumors. IHC staining for protein kinase C theta and DOG1 are helpful in distinguishing GIST from other mesenchymal tumors, particularly those that are KIT-negative. DOG1 (Discovered On GIST 1) is a protein of unknown function that is expressed strongly in GISTs and is rarely expressed in other mesenchymal neoplasms. KIT mutations in GISTs occur in Exon 9 (10%), Exon 11 (67%), Exon 13 (1%) and Exon 17 (1%). About 5% of the GISTs have activating mutation in the Platelet-Derived Growth Factor Receptor alpha (PDGFRA) gene which encodes for another tyrosine kinase receptor. Approximately 10% to 20% of GISTs have no KIT or PDGFRA mutations and are referred as wild-type GISTs. Mutational status is important as they may predict response to GLEEVEC® and also have prognostic significance. Patients with KIT exon 11 mutations are most sensitive and have a much higher response to GLEEVEC® whereas those with KIT exon 9 mutation or wild-type c-KIT may require a higher dose of GLEEVEC® (800 mg daily dose). KIT exon 11 mutations affecting the codons 557 and 558 is an independent adverse prognostic factor and associated with higher incidence of metastases whereas GISTs with KIT exon 9 mutations usually arise in the small bowel and are associated with frequent recurrences. GISTs with PDGFRA mutations in general have low mitotic count and low malignant potential and those with PDGFRA exon 18 mutation have favorable survival outcomes and located in the stomach. They are however resistant to GLEEVEC®. Although mutated KIT and PDGFRA have been identified as important driver mutations for GIST oncogenesis, the clinical significance of their single mutations has been unclear. To address this, the authors in this study identified 3067 patients with GIST from databases who had macroscopically complete tumor excision and had no detectable metastases at the time of diagnosis. Information on mutation analysis was available on 1505 tumors. The researchers then analyzed associations between KIT and PDGFRA mutations and Recurrence Free Survival (RFS) in this patient population treated with surgery alone.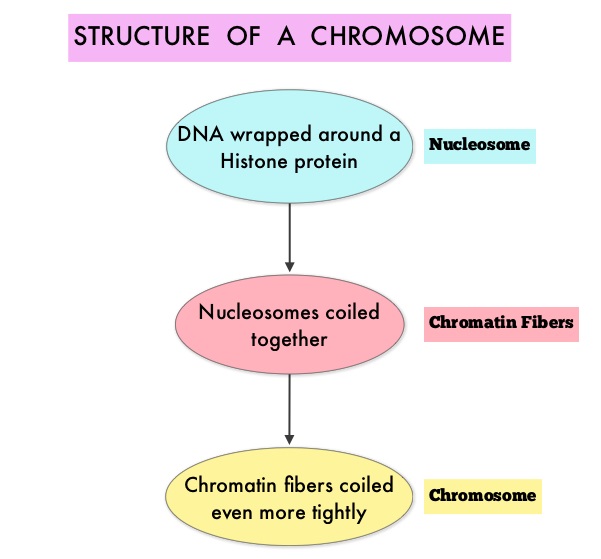 To briefly summarize the structure of a chromosome, individual loops of coiled double-helix DNA wrap around a histone protein to form a nucleosome. Nucleosomes are then coiled together to form chromatin fibers, which looks like beads on a string. The chromatin fibers are coiled even more tightly to form chromosomes. HDAC enzymes catalyze the removal of acetyl groups and regulate the level of acetylation of the histones and non-histone proteins and transcription of several genes. Hypoacetylation of histones has been associated with a condensed chromatin structure that results in the repression of gene transcription, whereas acetylated histones are associated with a more open chromatin structure and activation of gene transcription. HDACs are grouped into four major classes (Class I, II, III and IV) and regulate cell-cycle progression, cell survival, angiogenesis and immunity. The HDAC Class I enzymes are HDAC1, 2, 3 & 8 and are typically found in the nucleus where they are able to repress transcription. The HDAC Class II enzymes include HDAC4, 5, 6, 7, 9 and 10 and are able to move between the cytoplasm and nucleus and function in signal transduction. In Multiple Myeloma, the important enzyme to target is HDAC6.
To briefly summarize the structure of a chromosome, individual loops of coiled double-helix DNA wrap around a histone protein to form a nucleosome. Nucleosomes are then coiled together to form chromatin fibers, which looks like beads on a string. The chromatin fibers are coiled even more tightly to form chromosomes. HDAC enzymes catalyze the removal of acetyl groups and regulate the level of acetylation of the histones and non-histone proteins and transcription of several genes. Hypoacetylation of histones has been associated with a condensed chromatin structure that results in the repression of gene transcription, whereas acetylated histones are associated with a more open chromatin structure and activation of gene transcription. HDACs are grouped into four major classes (Class I, II, III and IV) and regulate cell-cycle progression, cell survival, angiogenesis and immunity. The HDAC Class I enzymes are HDAC1, 2, 3 & 8 and are typically found in the nucleus where they are able to repress transcription. The HDAC Class II enzymes include HDAC4, 5, 6, 7, 9 and 10 and are able to move between the cytoplasm and nucleus and function in signal transduction. In Multiple Myeloma, the important enzyme to target is HDAC6.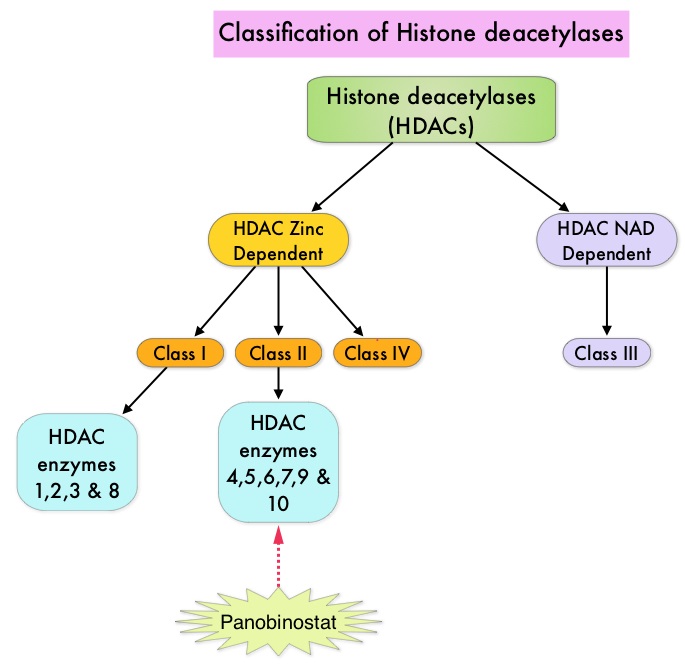 FARYDAK® is an oral, pan-histone deacetylase inhibitor which inhibits cell cycle progression and ultimately results in apoptosis. FARYDAK® inhibits the aggresome pathway of protein degradation which is upregulated when proteosome pathway is inhibited by VELCADE®. Based on preclinical data demonstrating synergy between VELCADE® and FARYDAK® in Myeloma, the PANORAMA 1 trial, enrolled patients with relapsed or refractory Multiple Myeloma who had received one to three prior lines of therapy and were not VELCADE® refractory. In this phase III trial, patients were randomly assigned to receive either FARYDAK® (N=387) or placebo (N=381), each along with IV VELCADE® and oral Dexamethasone. In this study, treatment was given in two 24 week phases. The first 24 week treatment phase was cycles 1 thru 8, where patients received placebo or FARYDAK® 20 mg orally QD 3 times a week for 2 weeks of a 3 week cycle; VELCADE® 1.3 mg/m2 IV twice weekly for 2 weeks of a 3 week cycle and Dexamethasone 20 mg PO on the day of and day after VELCADE®. Patients with clinical benefit (defined as complete response, partial response or stable disease, without significant toxicities) after the first eight cycles could proceed to the second phase of treatment in which FARYDAK® and Dexamethasone administration schedule remained the same but VELCADE® was administered once weekly for 2 weeks of the 3 week cycle. The median age was 63 years, 48% of patients had received at least two lines of therapy and 57% of patients had prior autologous stem cell transplantation and 43% had prior therapy with VELCADE®. The primary endpoint was Progression Free Survival (PFS) and secondary endpoints included Overall Survival (OS), Overall Response Rate (ORR), near Complete/Complete Response (nCR/CR) rate, Duration of Response (DOR), and safety. Among the patients enrolled in the FARYDAK® group (N = 387), 44% completed first phase of treatment and 26% completed the second phase of treatment. After a median follow up of 28 months, the primary end point of the study was met with a 37% decrease in the risk of disease progression in all the FARYDAK® group of patients compared to the placebo group (12 months vs 8.1 months, HR=0.63, P<0.0001). The median PFS was 14.65 months for those in the FARYDAK® group who completed the first phase of treatment and 17.64 months for those who completed the second phase of treatment. With regards to the secondary endpoints in the FARYDAK® vs placebo groups, the ORR was 60.7% vs 54.6% (P=0.87), nCR/CR rate was 27.6% vs 15.7% (P=0.00006), median duration of response was13.1months vs 10.9 months and median time to progression was 12.7 months vs 8.5 months respectively. It was noted that the nCR/CR rate was 52.9% for those patients who completed the second phase of treatment. The most common grade 3/4 adverse events in the FARYDAK® vs placebo arms included thrombocytopenia (67% vs 31%), neutropenia (35% vs 11%), and diarrhea (26% vs 8%) and these toxicities were manageable with dose reduction and supportive care. The authors concluded that a combination of FARYDAK®, VELCADE® and Dexamethasone significantly improves Progression Free Survival in patients with relapsed and refractory Multiple Myeloma, with manageable toxicities. Miguel JS, Hungria VTM , Yoon S, et al. 56th ASH Annual Meeting and Exposition, 2014. Abstract#4742
FARYDAK® is an oral, pan-histone deacetylase inhibitor which inhibits cell cycle progression and ultimately results in apoptosis. FARYDAK® inhibits the aggresome pathway of protein degradation which is upregulated when proteosome pathway is inhibited by VELCADE®. Based on preclinical data demonstrating synergy between VELCADE® and FARYDAK® in Myeloma, the PANORAMA 1 trial, enrolled patients with relapsed or refractory Multiple Myeloma who had received one to three prior lines of therapy and were not VELCADE® refractory. In this phase III trial, patients were randomly assigned to receive either FARYDAK® (N=387) or placebo (N=381), each along with IV VELCADE® and oral Dexamethasone. In this study, treatment was given in two 24 week phases. The first 24 week treatment phase was cycles 1 thru 8, where patients received placebo or FARYDAK® 20 mg orally QD 3 times a week for 2 weeks of a 3 week cycle; VELCADE® 1.3 mg/m2 IV twice weekly for 2 weeks of a 3 week cycle and Dexamethasone 20 mg PO on the day of and day after VELCADE®. Patients with clinical benefit (defined as complete response, partial response or stable disease, without significant toxicities) after the first eight cycles could proceed to the second phase of treatment in which FARYDAK® and Dexamethasone administration schedule remained the same but VELCADE® was administered once weekly for 2 weeks of the 3 week cycle. The median age was 63 years, 48% of patients had received at least two lines of therapy and 57% of patients had prior autologous stem cell transplantation and 43% had prior therapy with VELCADE®. The primary endpoint was Progression Free Survival (PFS) and secondary endpoints included Overall Survival (OS), Overall Response Rate (ORR), near Complete/Complete Response (nCR/CR) rate, Duration of Response (DOR), and safety. Among the patients enrolled in the FARYDAK® group (N = 387), 44% completed first phase of treatment and 26% completed the second phase of treatment. After a median follow up of 28 months, the primary end point of the study was met with a 37% decrease in the risk of disease progression in all the FARYDAK® group of patients compared to the placebo group (12 months vs 8.1 months, HR=0.63, P<0.0001). The median PFS was 14.65 months for those in the FARYDAK® group who completed the first phase of treatment and 17.64 months for those who completed the second phase of treatment. With regards to the secondary endpoints in the FARYDAK® vs placebo groups, the ORR was 60.7% vs 54.6% (P=0.87), nCR/CR rate was 27.6% vs 15.7% (P=0.00006), median duration of response was13.1months vs 10.9 months and median time to progression was 12.7 months vs 8.5 months respectively. It was noted that the nCR/CR rate was 52.9% for those patients who completed the second phase of treatment. The most common grade 3/4 adverse events in the FARYDAK® vs placebo arms included thrombocytopenia (67% vs 31%), neutropenia (35% vs 11%), and diarrhea (26% vs 8%) and these toxicities were manageable with dose reduction and supportive care. The authors concluded that a combination of FARYDAK®, VELCADE® and Dexamethasone significantly improves Progression Free Survival in patients with relapsed and refractory Multiple Myeloma, with manageable toxicities. Miguel JS, Hungria VTM , Yoon S, et al. 56th ASH Annual Meeting and Exposition, 2014. Abstract#4742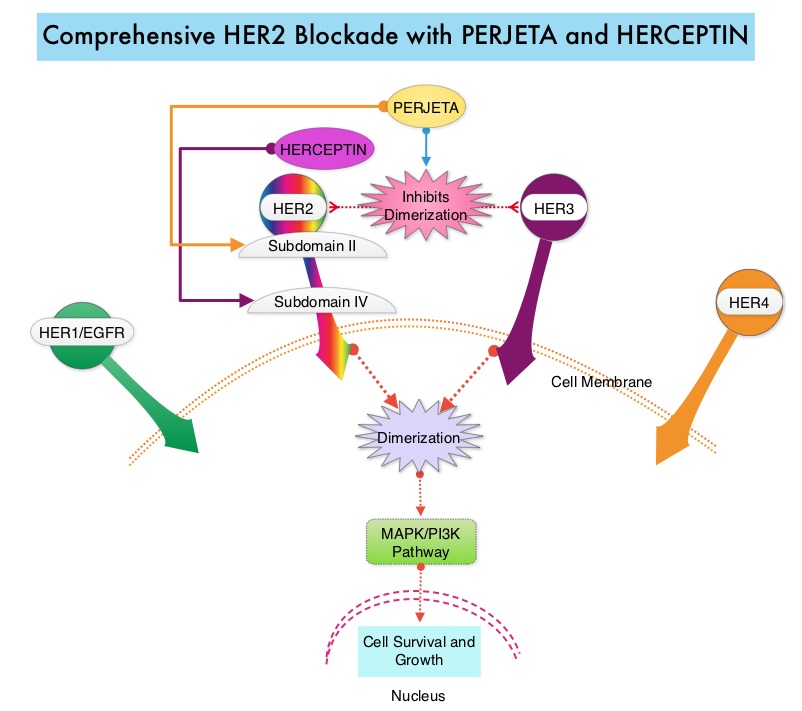 PERJETA® (Pertuzumab) is a recombinant humanized monoclonal antibody that binds to the HER2 at a different epitope of the HER2 extracellular domain (subdomain II) compared to HERCEPTIN® and prevents the dimerization of HER2 with HER3 receptor. PERJETA® stimulates antibody-dependent, cell-mediated cytotoxicity similar to HERCEPTIN®. By combining HERCEPTIN® and PERJETA®, a more comprehensive blockade of HER2 signaling can be accomplished, as these two agents bind to different HER2 epitopes and may complement each other and improve efficacy, as was demonstrated in early phase trials. The CLEOPATRA trial is a phase III study in which 808 treatment naive HER positive metastatic breast cancer patients, were randomly assigned to receive either HERCEPTIN® plus Docetaxel or this two drug combination given along with PERJETA®. PERJETA® was given as an 840 mg loading dose followed by a 420 mg maintenance dose, HERCEPTIN® was given as an 8 mg/kg loading dose followed by a 6 mg/kg maintenance dose and Docetaxel was given at 75 mg/m2 for at least 6 cycles. Treatment was administered every 3 weeks and continued until disease progression. The primary end point of this study was Progression Free Survival and secondary end points included Overall Survival, Objective Response Rate and safety. A previous analysis performed in May 2012 showed that the addition of PERJETA® to the combination of HERCEPTIN® and Docetaxel significantly prolonged Progression Free Survival compared to HERCEPTIN® plus Docetaxel alone (18.5 months vs 12.4 months) but the median overall survival had not been reached then. In this final Overall Survival analysis, at a median follow up of 50 months, median Overall Survival was 56.5 months with the PERJETA® combination compared to 40.8 months in the non-PERJETA® group (hazard ratio [HR] = 0.68; P<0.001). This meant that adding PERJETA® to HERCEPTIN® and Docetaxel increased the median Overall Survival by 15.7 months. The increase in Progression Free Survival by 6.3 months with the PERJETA® combination, was again maintained (HR = 0.68, P < 0.0001), at the time of the final analysis. The median duration of response was 20.2 months with the PERJETA® combination compared to 12.5 months in the non-PERJETA® group. The incidence of symptomatic left ventricular dysfunction as well as declines in left ventricular ejection fraction, were rare and similar between the two treatment groups. Based on the CLEOPATRA study, women with HER positive metastatic breast cancer should be considered candidates, for treatment with a combination of PERJETA®, HERCEPTIN® and Docetaxel. Swain S, Baselga J, Kim S, et al. N Engl J Med 2015; 372:724-734
PERJETA® (Pertuzumab) is a recombinant humanized monoclonal antibody that binds to the HER2 at a different epitope of the HER2 extracellular domain (subdomain II) compared to HERCEPTIN® and prevents the dimerization of HER2 with HER3 receptor. PERJETA® stimulates antibody-dependent, cell-mediated cytotoxicity similar to HERCEPTIN®. By combining HERCEPTIN® and PERJETA®, a more comprehensive blockade of HER2 signaling can be accomplished, as these two agents bind to different HER2 epitopes and may complement each other and improve efficacy, as was demonstrated in early phase trials. The CLEOPATRA trial is a phase III study in which 808 treatment naive HER positive metastatic breast cancer patients, were randomly assigned to receive either HERCEPTIN® plus Docetaxel or this two drug combination given along with PERJETA®. PERJETA® was given as an 840 mg loading dose followed by a 420 mg maintenance dose, HERCEPTIN® was given as an 8 mg/kg loading dose followed by a 6 mg/kg maintenance dose and Docetaxel was given at 75 mg/m2 for at least 6 cycles. Treatment was administered every 3 weeks and continued until disease progression. The primary end point of this study was Progression Free Survival and secondary end points included Overall Survival, Objective Response Rate and safety. A previous analysis performed in May 2012 showed that the addition of PERJETA® to the combination of HERCEPTIN® and Docetaxel significantly prolonged Progression Free Survival compared to HERCEPTIN® plus Docetaxel alone (18.5 months vs 12.4 months) but the median overall survival had not been reached then. In this final Overall Survival analysis, at a median follow up of 50 months, median Overall Survival was 56.5 months with the PERJETA® combination compared to 40.8 months in the non-PERJETA® group (hazard ratio [HR] = 0.68; P<0.001). This meant that adding PERJETA® to HERCEPTIN® and Docetaxel increased the median Overall Survival by 15.7 months. The increase in Progression Free Survival by 6.3 months with the PERJETA® combination, was again maintained (HR = 0.68, P < 0.0001), at the time of the final analysis. The median duration of response was 20.2 months with the PERJETA® combination compared to 12.5 months in the non-PERJETA® group. The incidence of symptomatic left ventricular dysfunction as well as declines in left ventricular ejection fraction, were rare and similar between the two treatment groups. Based on the CLEOPATRA study, women with HER positive metastatic breast cancer should be considered candidates, for treatment with a combination of PERJETA®, HERCEPTIN® and Docetaxel. Swain S, Baselga J, Kim S, et al. N Engl J Med 2015; 372:724-734
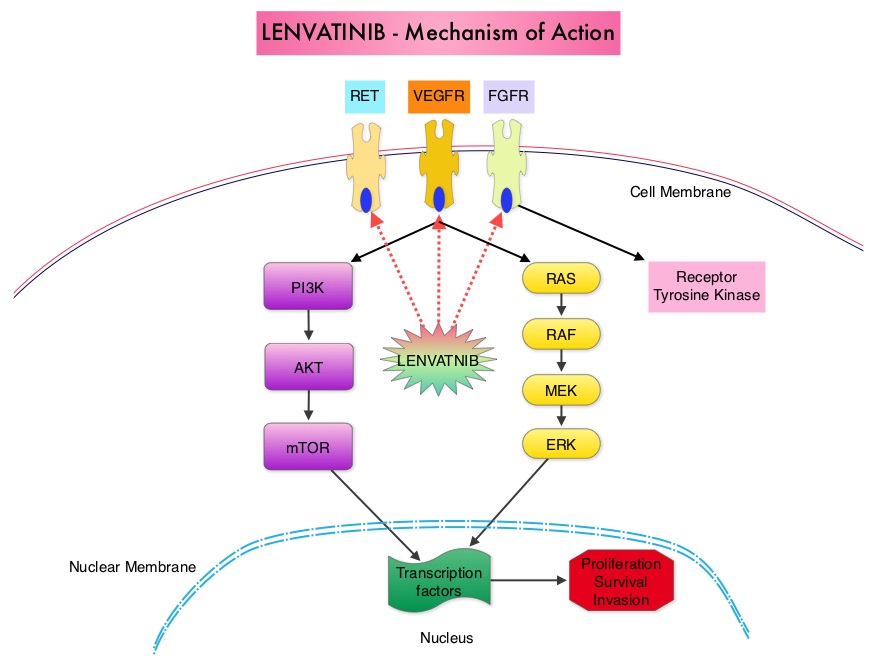 The discovery of genetic alterations in the MAP Kinase pathway as well as the PI3K (Phosphatidylinositol-3-Kinase)-AKT-mTOR pathway in thyroid tumors, has lead to the development of Tyrosine Kinase Inhibitors (TKI’s), to target these activated pathways. LENVIMA® is an oral multitargeted TKI which targets Vascular Endothelial Growth Factor Receptor (VEGFR)1-3, Fibroblast Growth factor Receptor (FGFR)1-4, Rearranged during Transfection tyrosine kinase receptor (RET), c-KIT, and Platelet Derived Growth Factor Receptor (PDGFR). LENVIMA® differs from other TKIs with antiangiogenesis properties by its ability to inhibit FGFR-1 thereby blocking the mechanisms of resistance to VEGF/VEGFR inhibitors. In addition, it controls tumor cell growth by inhibiting RET, c-KIT, and PDGFR beta and influences tumor microenvironment by inhibiting by FGFR and PDGFR beta.
The discovery of genetic alterations in the MAP Kinase pathway as well as the PI3K (Phosphatidylinositol-3-Kinase)-AKT-mTOR pathway in thyroid tumors, has lead to the development of Tyrosine Kinase Inhibitors (TKI’s), to target these activated pathways. LENVIMA® is an oral multitargeted TKI which targets Vascular Endothelial Growth Factor Receptor (VEGFR)1-3, Fibroblast Growth factor Receptor (FGFR)1-4, Rearranged during Transfection tyrosine kinase receptor (RET), c-KIT, and Platelet Derived Growth Factor Receptor (PDGFR). LENVIMA® differs from other TKIs with antiangiogenesis properties by its ability to inhibit FGFR-1 thereby blocking the mechanisms of resistance to VEGF/VEGFR inhibitors. In addition, it controls tumor cell growth by inhibiting RET, c-KIT, and PDGFR beta and influences tumor microenvironment by inhibiting by FGFR and PDGFR beta. 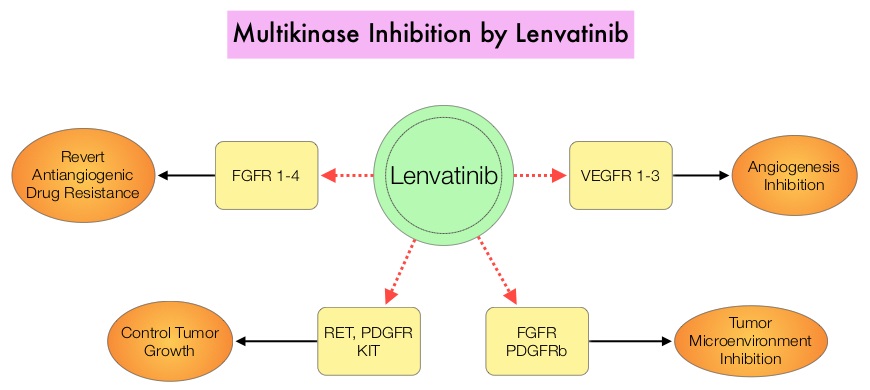 The SELECT trial is a double-blind, multicenter, phase III study in which 392 patients with advanced RAI-refractory Differentiated Thyroid Cancer (DTC) were randomly assigned in a 2:1 ratio to receive LENVIMA® 24 mg PO daily in 28-day cycles (N=261) or placebo (N=131). Both treatment groups were well balanced and pretreatment with one prior Tyrosine Kinase Inhibitor (TKI) was allowed. Patients in the placebo group were allowed to cross over and receive open-label LENVIMA®, at the time of disease progression. The primary end point was Progression Free Survival (PFS) and secondary end points included Response Rate, Overall Survival, and safety. The median Progression Free Survival was 18.3 months in the LENVIMA® group and 3.6 months in the placebo group (HR= 0.21; P<0.001). This benefit in PFS associated with LENVIMA® was observed in all pre-specified subgroup of patients including those who had received one prior treatment with a TKI. The objective response rate with LENVIMA® was 64.8% versus 1.5% with placebo (P<0.001) and the median overall survival was not reached in either group. The most frequently reported grade 3 or more adverse events in the LENVIMA® group included hypertension (42.9%) and proteinuria (10%). Approximately 14% of the patients in the LENVIMA® group discontinued the drug due to adverse effects. Exploratory biomarker analyses were performed for BRAF and RAS mutations on tumor tissue and it was noted that LENVIMA® benefitted patients regardless of BRAFor RAS mutation status. The authors concluded that LENVIMA® decreased the risk of disease progression by 79% as compared with placebo and was associated with significant improvements in objective Response Rate among patients with RAI-refractory thyroid cancer. NEXAVAR® (Sorafenib), another multitargeted TKI, is presently available for this group of patients and therefore proper sequencing of LENVIMA® and NEXAVAR® remains unknown although it appears that LENVIMA® has a markedly higher Progression Free Survival compared to NEXAVAR®. Schlumberger M, Tahara M, Wirth LJ, et al. N Engl J Med 2015; 372:621-630
The SELECT trial is a double-blind, multicenter, phase III study in which 392 patients with advanced RAI-refractory Differentiated Thyroid Cancer (DTC) were randomly assigned in a 2:1 ratio to receive LENVIMA® 24 mg PO daily in 28-day cycles (N=261) or placebo (N=131). Both treatment groups were well balanced and pretreatment with one prior Tyrosine Kinase Inhibitor (TKI) was allowed. Patients in the placebo group were allowed to cross over and receive open-label LENVIMA®, at the time of disease progression. The primary end point was Progression Free Survival (PFS) and secondary end points included Response Rate, Overall Survival, and safety. The median Progression Free Survival was 18.3 months in the LENVIMA® group and 3.6 months in the placebo group (HR= 0.21; P<0.001). This benefit in PFS associated with LENVIMA® was observed in all pre-specified subgroup of patients including those who had received one prior treatment with a TKI. The objective response rate with LENVIMA® was 64.8% versus 1.5% with placebo (P<0.001) and the median overall survival was not reached in either group. The most frequently reported grade 3 or more adverse events in the LENVIMA® group included hypertension (42.9%) and proteinuria (10%). Approximately 14% of the patients in the LENVIMA® group discontinued the drug due to adverse effects. Exploratory biomarker analyses were performed for BRAF and RAS mutations on tumor tissue and it was noted that LENVIMA® benefitted patients regardless of BRAFor RAS mutation status. The authors concluded that LENVIMA® decreased the risk of disease progression by 79% as compared with placebo and was associated with significant improvements in objective Response Rate among patients with RAI-refractory thyroid cancer. NEXAVAR® (Sorafenib), another multitargeted TKI, is presently available for this group of patients and therefore proper sequencing of LENVIMA® and NEXAVAR® remains unknown although it appears that LENVIMA® has a markedly higher Progression Free Survival compared to NEXAVAR®. Schlumberger M, Tahara M, Wirth LJ, et al. N Engl J Med 2015; 372:621-630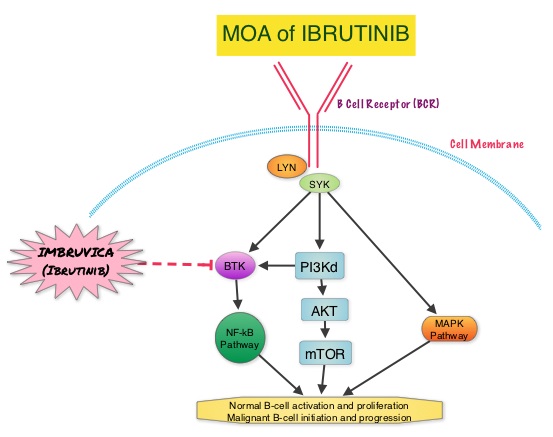 About 90% of patients with Waldenstrom Macroglobulinemia demonstrate a mutation in chromosome 3p (MYD88 L265P), which is specific to WM and may be an early oncogenic event in WM pathogenesis.. It appears that MYD88 L265P promotes malignant cell proliferation via the Bruton’s Tyrosine Kinase (BTK) signaling pathway. Mutations in CXCR4 gene are present in 30% of patients with WM, and their expression induces BTK activity and may confer resistance to BTK inhibitors. IMBRUVICA® (Ibrutinib) is an oral, irreversible inhibitor of BTK and inhibits cell proliferation and promotes programmed cell death (Apoptosis). Preliminary studies in WM patients have revealed that IMBRUVICA® prevents the binding of MYD88 L265P (mutated gene) to BTK thereby selectively killing tumor cells. On the other hand it was noted that a major response to IMBRUVICA® was less likely when mutations in CXCR4 gene were present in the tumor cells. With this molecular understanding of WM, the authors enrolled 63 patients with relapsed/refractory symptomatic WM and were treated with IMBRUVICA®, 420 mg PO daily for 2 years or until disease progression or unacceptable toxicity. Anemia was main indication for treatment initiation (87.3% of patients) and the mean baseline hemoglobin level was 10.5 g/dL, mean serum IgM level was 3610 mg/dL, 70% had bone marrow involvement and 60% of patients had lymphadenopathy.
About 90% of patients with Waldenstrom Macroglobulinemia demonstrate a mutation in chromosome 3p (MYD88 L265P), which is specific to WM and may be an early oncogenic event in WM pathogenesis.. It appears that MYD88 L265P promotes malignant cell proliferation via the Bruton’s Tyrosine Kinase (BTK) signaling pathway. Mutations in CXCR4 gene are present in 30% of patients with WM, and their expression induces BTK activity and may confer resistance to BTK inhibitors. IMBRUVICA® (Ibrutinib) is an oral, irreversible inhibitor of BTK and inhibits cell proliferation and promotes programmed cell death (Apoptosis). Preliminary studies in WM patients have revealed that IMBRUVICA® prevents the binding of MYD88 L265P (mutated gene) to BTK thereby selectively killing tumor cells. On the other hand it was noted that a major response to IMBRUVICA® was less likely when mutations in CXCR4 gene were present in the tumor cells. With this molecular understanding of WM, the authors enrolled 63 patients with relapsed/refractory symptomatic WM and were treated with IMBRUVICA®, 420 mg PO daily for 2 years or until disease progression or unacceptable toxicity. Anemia was main indication for treatment initiation (87.3% of patients) and the mean baseline hemoglobin level was 10.5 g/dL, mean serum IgM level was 3610 mg/dL, 70% had bone marrow involvement and 60% of patients had lymphadenopathy.  Sanger sequencing was used to determine MYD88 and CXCR4 mutations in the bone marrow lymphoplasmacytic cells. At best response, the median serum IgM levels declined to 1340 mg/dL (P<0.00001), median hemoglobin rose to 12.6 g/dL, (P<0.00001). At 6 months, bone marrow assessment post treatment, demonstrated a reduction in WM disease involvement from 70% to 45% (P=0.0006). With a median follow up at 6 cycles, the best overall response rate was 81% and a median time to response was 4 weeks. In patients who underwent tumor sequencing, mutations in CXCR4 gene impacted response rates. The major response rate for patients with wild-type CXCR4 gene was 77% compares to 30% in those with CXCR4 mutations (p=0.018). Further, patients with wild-type CXCR4 also had increased peripheral lymphocytosis following treatment with IMBRUVICA® compared to those with CXCR4 mutations (P=0.001). The most common more than grade 2 treatment related toxicities included thrombocytopenia (14.3%) and neutropenia (19.1%). The authors concluded that IMBRUVICA® is highly active in patients with relapsed or refractory Waldenstrom Macroglobulinemia, with rapid reductions in serum IgM level and improved hemoglobin levels. The presence of CXCR4 mutations negatively impact response rates in this patient group. Treon SP, Tripsas CK, Yang G, et al. Presented at: 55th ASH Annual Meeting; December 7-10, 2013; New Orleans, LA. Abstract 251.
Sanger sequencing was used to determine MYD88 and CXCR4 mutations in the bone marrow lymphoplasmacytic cells. At best response, the median serum IgM levels declined to 1340 mg/dL (P<0.00001), median hemoglobin rose to 12.6 g/dL, (P<0.00001). At 6 months, bone marrow assessment post treatment, demonstrated a reduction in WM disease involvement from 70% to 45% (P=0.0006). With a median follow up at 6 cycles, the best overall response rate was 81% and a median time to response was 4 weeks. In patients who underwent tumor sequencing, mutations in CXCR4 gene impacted response rates. The major response rate for patients with wild-type CXCR4 gene was 77% compares to 30% in those with CXCR4 mutations (p=0.018). Further, patients with wild-type CXCR4 also had increased peripheral lymphocytosis following treatment with IMBRUVICA® compared to those with CXCR4 mutations (P=0.001). The most common more than grade 2 treatment related toxicities included thrombocytopenia (14.3%) and neutropenia (19.1%). The authors concluded that IMBRUVICA® is highly active in patients with relapsed or refractory Waldenstrom Macroglobulinemia, with rapid reductions in serum IgM level and improved hemoglobin levels. The presence of CXCR4 mutations negatively impact response rates in this patient group. Treon SP, Tripsas CK, Yang G, et al. Presented at: 55th ASH Annual Meeting; December 7-10, 2013; New Orleans, LA. Abstract 251.