SUMMARY: The FDA on August 30, 2017, granted regular approval to KYMRIAH® (Tisagenlecleucel) for the treatment of patients up to age 25 years with B-cell precursor Acute Lymphoblastic Leukemia (ALL), that is refractory or in second or later relapse. KYMRIAH® is the first Chimeric Antigen Receptor (CAR) T-cell immunotherapy approved by the FDA.
What is (CAR) T-cell immunotherapy? Chimeric Antigen Receptor (CAR) T-cell therapy is a type of immunotherapy and consists of T cells collected from the patient’s blood in a leukapheresis procedure, and genetically engineered to produce special receptors on their surface called Chimeric Antigen Receptors (CAR). These reprogrammed cytotoxic T cells with the Chimeric Antigen Receptors on their surface are now able to recognize a specific antigen on tumor cells. These genetically engineered and reprogrammed CAR T-cells are grown in the lab and are then infused into the patient. These cells in turn proliferate in the patient’s body and the engineered receptor on the cell surface help recognize and kill cancer cells that expresses that specific antigen. KYMRIAH® (genetically engineered T-cells) seeks out cancer cells expressing the antigen CD19, which is found uniquely on B cells and destroy them. Patients, following treatment with CAR T-cells, develop B-cell aplasia (absence of CD19 positive cells) due to B-cell destruction and may need immunoglobin replacement. Hence, B-cell aplasia can be a useful therapeutic marker, as continued B-cell aplasia has been seen in all patients who had sustained remission, following CAR T-cell therapy.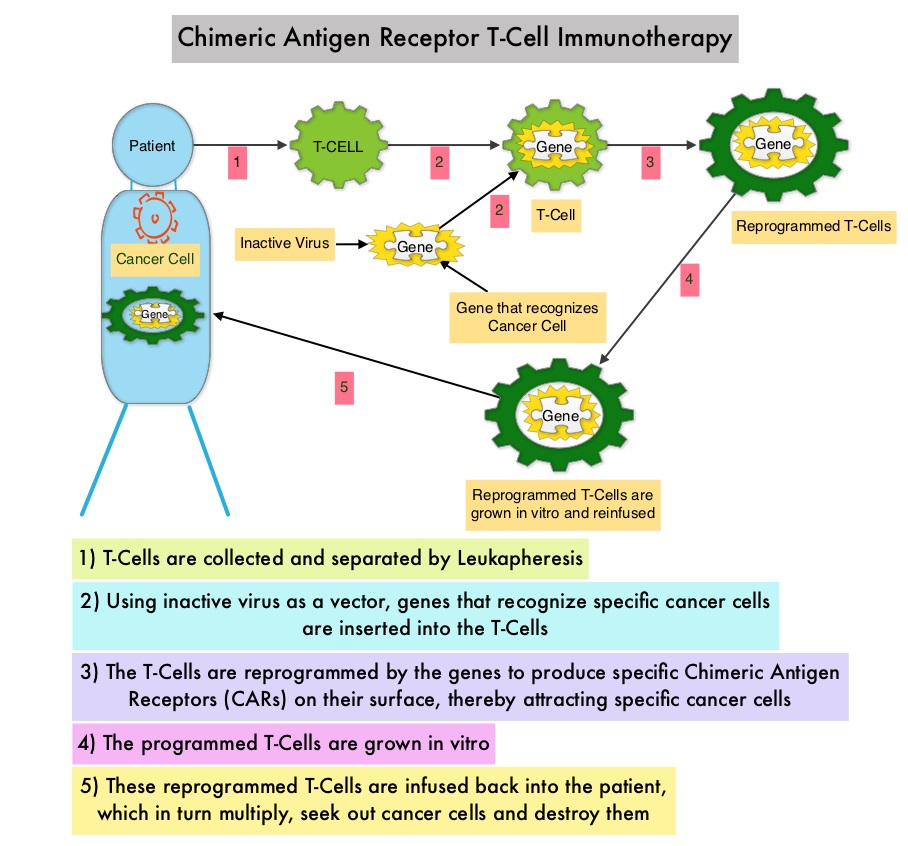 Cytokine Release Syndrome, an inflammatory process is the most common and serious side effect of CAR T-cell therapy and is associated with marked elevation of Interleukin-6. Cytokine release is important for T-cell activation and can result in high fevers and myalgias. This is usually self limiting although if severe can be associated with hypotension and respiratory insufficiency. Tocilizumab (ACTEMRA®), an Interleukin-6 receptor blocking antibody produces a rapid improvement in symptoms. This is however not recommended unless the symptoms are severe and life threatening, as blunting the cytokine response can in turn negate T-cell proliferation. Elevated serum Ferritin and C-reactive protein levels are surrogate markers for severe Cytokine Release Syndrome.
Cytokine Release Syndrome, an inflammatory process is the most common and serious side effect of CAR T-cell therapy and is associated with marked elevation of Interleukin-6. Cytokine release is important for T-cell activation and can result in high fevers and myalgias. This is usually self limiting although if severe can be associated with hypotension and respiratory insufficiency. Tocilizumab (ACTEMRA®), an Interleukin-6 receptor blocking antibody produces a rapid improvement in symptoms. This is however not recommended unless the symptoms are severe and life threatening, as blunting the cytokine response can in turn negate T-cell proliferation. Elevated serum Ferritin and C-reactive protein levels are surrogate markers for severe Cytokine Release Syndrome.
The CAR T-cells have been shown to also access sanctuary sites such as the central nervous system and eradicate cancer cells. CD19 antigen is expressed by majority of the B cell malignancies and therefore most studies using CAR T-cell therapy have focused on the treatment of advanced B-cell malignancies such as Chronic Lymphocytic Leukemia (CLL), Acute Lymphoblastic Leukemia (ALL) and Non Hodgkin lymphoma (NHL), such as Diffuse Large B-Cell Lymphoma (DLBCL). Previously published studies have shown significant responses with CAR T-cell therapy in patients with relapsed and refractory B-cell ALL. But the durability of remission has remained unclear.
This FDA approval was based on ELIANA, an ongoing single-arm, open-label, multicenter, global, phase II study, that has enrolled 88 pediatric/young adult patients with CD19-positive Relapsed/Refractory B-cell ALL and with 5% or more bone marrow lymphoblasts by morphology. The median age was 12 years, 59% of patients had prior allogeneic Stem Cell Transplant and patients had received a median of 3 lines of therapy previously. Following completion of lymphodepleting chemotherapy which in most patients consisted of Fludarabine/Cyclophosphamide, patients were infused with a single dose of KYMRIAH® within 2 to 14 days following the completion of lymphodepleting chemotherapy. The primary endpoint was Overall Remission Rate – Complete Remission [CR] + CR with incomplete blood count recovery [CRi], within 3 months. Secondary endpoints included Duration of Remission (DOR), Overall Survival, Safety, and cellular kinetics.
The authors reported an updated interim analysis with a median study follow up of 6.4 months. Of the 63 patients who were evaluable for efficacy, the Overall Remission Rate was 83%, with 63% of patients achieving a Complete Remission (CR) and 19% achieving a Complete Remission with incomplete hematological recovery (CRi) within 3 months of KYMRIAH® infusion. All patients with a confirmed CR or CRi were Minimal Residual Disease (MRD) negative by flow cytometry. The relapse-free probability at 6 months after remission onset was 75% and the median Duration of Remission has not been reached. The probability of survival at 6 months was 89% and at 12 months was 79%. Thirteen percent (13%) of responders went on to have allogeneic Stem Cell Transplant within 6 months, while in remission.
The most common adverse reactions were Cytokine Release Syndrome (CRS), hypogammaglobulinemia, nausea, diarrhea, vomiting, fever, infections, headache, encephalopathy, hypotension, bleeding episodes, tachycardia, hypoxia and fatigue. Grade 3 or 4 adverse events were noted in 84% of patients. Patients with Severe Cytokine Release Syndrome were effectively treated with the Interleukin-6 receptor blocking antibody Tocilizumab (ACTEMRA®). The expansion of the genetically engineered T-cells in vivo correlated with CRS severity, and persistence of these reprogrammed T cells along with B-cell aplasia in peripheral blood was observed for 1 year or more in some responders.
The authors concluded that the ELIANA trial is the first multicenter trial of a CAR T-cell therapy and a single infusion of KYMRIAH® was highly efficacious, in patients with relapsed and refractory ALL, and was associated with a high and durable remission rate. This technology may be applied to other malignancies, as new antigen targets are identified. Buechner J, Grupp SA, Maude SL, et al. Global registration trial of efficacy and safety of CTL019 in pediatric and young adult patients with relapsed/refractory (r/r) acute lymphoblastic leukemia (ALL): update to the interim analysis. Presented at: 2017 EHA Congress; June 22-25, 2017; Madrid, Spain. Abstract S476.

