SUMMARY: The American Cancer Society estimates that approximately 20,110 new cases of Chronic Lymphocytic Leukemia (CLL) will be diagnosed in 2017 and approximately 4660 patients will die from the disease. CLL is a heterogeneous disease with a clinical course that is variable, with a very indolent course in some patients and some with aggressive disease and others somewhere in between. Both Binet and the Rai CLL staging systems developed in the 1970’s rely solely on physical examination and standard laboratory testing to predict survival. With the development of Interphase Fluorescent In Situ Hybridization (FISH) technique, which allows detection of genetic abnormalities in noncycling CLL cells, it has become clear that cytogenetic abnormalities are often seen in CLL patients and these genetic abnormalities in turn appear to be reliable predictors of disease progression, response to therapy and survival. Some of these cytogenetic abnormalities include del(13q), normal karyotype, trisomy(12), del(11q), del(17p), and they are associated with decreasing survival times, in that order. Another important prognostic factor is the rearrangement and somatic hypermutation of the variable region of the immunoglobulin heavy chain genes (IGHV), which is an independent predictor of outcome in CLL. Retrospective studies have suggested that patients with CLL whose leukemic cells unmutated IGHV gene demonstrated more aggressive disease and shorter survival time compared to those patients with somatic hypermutations in their IGHV genes (Mutated IGHV gene). Expression of two flow cytometry based biomarkers, CD38 (surface marker) and ZAP-70 (intracytoplasmic protein), have been associated with poor outcomes as well.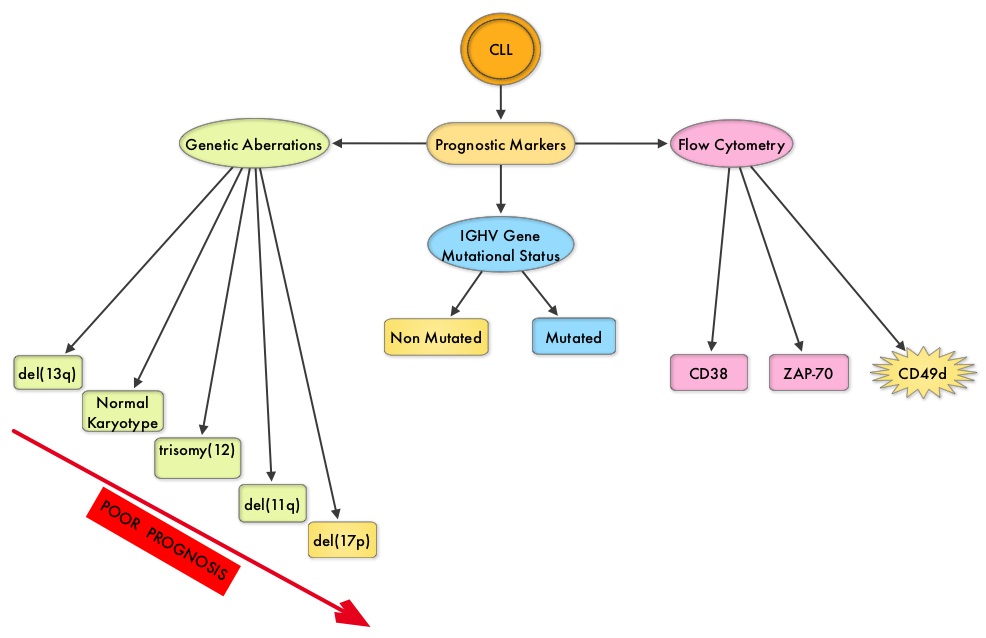
The rationale for maintenance treatment in the CLL patients is based on previously published studies showing that about 30% of patients with CLL did not substantially benefit from chemoimmunotherapy alone. These patients belong to the high risk group, as defined by poor cytogenetics, positive Minimal Residual Disease at the end of chemoimmunotherapy and unmutated IGHV gene status. The combined use of Genetic markers and Minimal Residual Disease (MRD) assessment can therefore identify patients with CLL, who have a poor outcome with first line chemoimmunotherapy.
Maintenance therapy with REVLIMID® (Lenalidomide)/Dexamethasone is considered standard of care for patients with multiple myeloma, regardless of transplantation and for newly diagnosed non-transplant candidates, as this intervention was found to improve Progression Free Survival significantly, with a favorable safety profile. Two phase III studies demonstrating the benefit of maintenance treatment with REVLIMID® in patients with CLL, were presented at the 2016 American Society of Hematology (ASH) Annual Meeting & Exposition. The first study (CLL M1 study) was conducted to demonstrate the value of maintenance treatment with REVLIMID® in patients with high risk CLL, following first line chemoimmunotherapy. For this study, 468 patients were screened, of whom 89 patients (N=89) were considered as high risk for disease progression, following 4 cycles of chemoimmunotherapy. This was based on the following factors: MRD levels of 10-2 or higher or MRD levels of 10-4 or higher to less than 10-2, combined with either an unmutated IGHV gene status, del(17p) or TP53 mutation at baseline.
Patients received investigator’s choice of chemoimmunotherapy regimens which included Fludarabine/Cyclophosphamide/Rituximab (FCR), Fludarabine and Rituximab (FR), Fludarabine and Cyclophosphamide (FC), or Bendamustine and Rituximab (BR). The most common regimens administered were Bendamustine and Rituximab followed by FCR. Approximately 78% of all patients reached Minimal Residual Disease negativity and these patients were ineligible for this trial. The 89 eligible patients were randomized in a 2:1 ratio to receive REVLIMID® maintenance (N=60) or Placebo (N=29). Treatment with REVLIMID® or Placebo was started at 5 mg daily for the first cycle, and was subsequently increased to the target dose of 15 mg by the 7th cycle. The median age of these patients was 64 years. At randomization, 37% of patients had a high and 63% had an intermediate MRD level, respectively. Based on their risk for thromboembolic events, patients received either low dose aspirin daily or appropriate prophylactic anticoagulants. The primary endpoint was Progression Free Survival (PFS).
After a median follow up of 17.7 months, the median PFS for REVLIMID® group has not been reached and was 13.3 months for the Placebo group. This meant a relative risk reduction for disease progression of 80% and this was highly statistically significant (P < 0.00001). There was no difference in Overall Survival at the time of this interim analysis. Adverse events such as neutropenia, diarrhea, skin disorders, etc. were more frequently noted with REVLIMID® maintenance treatment.
The authors concluded that REVLIMID® maintenance treatment for high risk CLL patients, after first line chemoimmunotherapy, significantly prolonged Progression Free Survival and this study confirmed the prognostic significance of the MRD based risk assessment.
The second study, CONTINUUM Trial, is a multicenter, randomized, double-blinded phase III study, designed to evaluate the efficacy and safety of REVLIMID® as maintenance therapy in previously treated CLL patients. In this study, 314 CLL patients who at least had a partial response (PR) to second line therapy were randomized 1:1 to receive either REVLIMID® 2.5 mg once daily on days 1-28 of the first 28 day cycle, or matching Placebo. If tolerated, REVLIMID® dose was then increased to 5 mg daily from cycle 2, and further increased to 10 mg daily at cycle 7 and thereafter. Co-primary endpoints were Progression Free Survival (PFS) and Overall Survival and secondary endpoints included Safety, Tumor response, Duration of Response and Health related Quality of Life measures.
At a median follow up of 31.5 months, the median PFS was significantly longer for the REVLIMID® group compared to the Placebo group (33.9 vs 9.2 months; HR=0.40; P<0.001). This benefit with REVLIMID® maintenance was maintained in all patient subgroups regardless of age, prior response to chemotherapy and number of poor prognostic factors.
The authors in this trial concluded that REVLIMID® maintenance resulted in a 60% reduction in the risk of disease progression in patients with CLL following second line treatment, and there was no meaningful difference in the Quality of Life between the two treatment groups.
Taken together, these two phase III studies suggest that REVLIMID® maintenance therapy following either first line or second line chemoimmunotherapy, significantly prolongs Progression Free Survival in patients with CLL. This strategy will however not be applicable to patients receiving upfront chemotherapy-free regimens such as BTK Inhibitor, IMBRUVICA® (Ibrutinib).
1. Lenalidomide maintenance after front line therapy substantially prolongs progression free survival in high risk CLL. Fink AM, Bahlo J, Sandra R, et al. 2016 ASH Annual Meeting. Abstract 229. Presented December 3, 2016.
2. Results of the phase 3 study of lenalidomide versus placebo as maintenance therapy following second-line treatment for patients with chronic lymphocytic leukemia. Foà R, Schuh A, Zaritskey A, et al. 2016 ASH Annual Meeting. Abstract 230. Presented December 3, 2016.

