SUMMARY: The FDA on May 18, 2016 approved TECENTRIQ® (Atezolizumab) for the treatment of patients with locally advanced or metastatic urothelial carcinoma, whose disease has worsened during or following platinum containing chemotherapy, or within 12 months of receiving platinum containing chemotherapy, either before (neoadjuvant) or after (adjuvant) surgical treatment. Urothelial carcinoma accounts for 90 percent of all bladder cancers and can originate in the renal pelvis, ureter and urethra. The National Cancer Institute estimates that in 2016, approximately 76,960 new cases of Bladder Cancer will be diagnosed and 16,390 patients will die of the disease. Treatment options for patients who progress after platinum based chemotherapy are limited, with poor outcomes. The treatment paradigm for solid tumors has been rapidly evolving, with a better understanding of the Immune checkpoints or gate keepers. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, Immune checkpoints or gate keepers inhibit an intense immune response by switching off the T cells of the immune system. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), as well as Programmed cell Death Ligands (PD-L1) that are expressed by cells in the tumor micro environment. By doing so, T cells are unleashed, resulting in T cell proliferation, activation and a therapeutic response.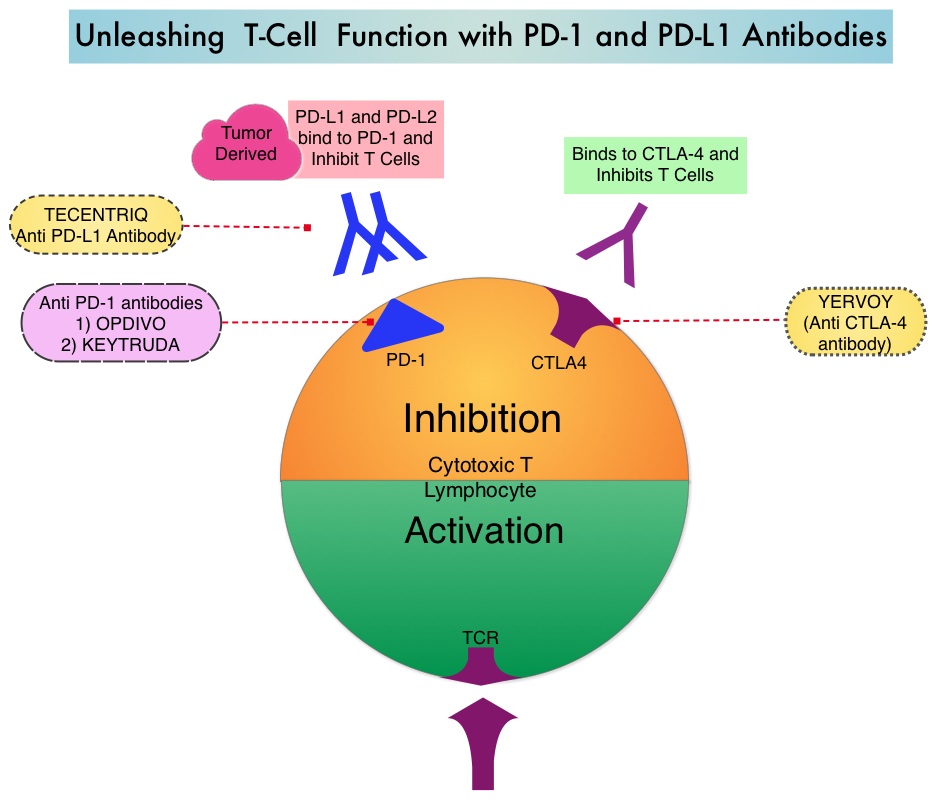
TECENTRIQ® (Atezolizumab) is an anti-PDL1 monoclonal antibody designed to directly bind to PD-L1 expressed on tumor cells and tumor-infiltrating Immune Cells, thereby blocking its interactions with PD-1 and B7.1 receptors and thus enabling the activation of T cells. The FDA approval was based on IMvigor 210 trial which is an open label, single arm, multicenter, phase II study in which the safety and efficacy of TECENTRIQ® was evaluated in patients with locally advanced or metastatic urothelial carcinoma, regardless of PD-L1 expression. In this study, 310 patients whose disease had progressed during or following previous treatment with a platinum based chemotherapy regimen received TECENTRIQ® 1200 mg as an intravenous dose once every 21 days cycles until disease progression. The primary endpoint of the study was Objective Response Rate (ORR) and secondary endpoints included Duration of Response (DOR), Overall Survival (OS), Progression Free Survival (PFS) and safety. PD-L1 expression on tumor-infiltrating Immune Cells (IC) was assessed prospectively by ImmunoHistoChemistry using Formalin-Fixed Paraffin-Embedded tumor specimens. PD-L1 positivity was defined as follows – IC 2/3 (5% or more), IC 1 (1-5%) and IC 0 (<1%).
In an updated analysis, with a median follow of 11.7 months, the ORR was 15% for the entire cohort of patients (P=0.0058). The ORR in the IC 2/3 group was 26% (P<0.0001) and 18% in the IC 1 group (P=0.0004). Ongoing responses were noted in 84% of the responding patients. The most common side effects associated with TECENTRIQ® included fatigue, decreased appetite, nausea, urinary tract infection, fever and constipation.
The authors concluded that TECENTRIQ® significantly improves Objective Response rate (ORR) in metastatic urothelial cancers and this benefit is even more so in those tumors with a higher level of PD-L1 expression. Rosenberg JE, Hoffman-Censits, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016; Published online 4 March. DOI: http://dx.doi.org/10.1016/S0140-6736(16)00561-4

