SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately, 246,660 new cases of invasive breast cancer will be diagnosed in 2016 and 40,450 women will die of the disease. Approximately 75% of patients with breast cancer are hormone receptor positive (Estrogen Receptor/Progesterone Receptor positive) and this is a predictor of response to endocrine therapy. In premenopausal woman, the ovary is the main source of estrogen production, whereas in postmenopausal women, the primary source of estrogen is the Aromatase enzyme mediated conversion of androstenedione and testosterone to estrone and estradiol in extragonadal/peripheral tissues. Presently available therapies include Tamoxifen and other Selective Estrogen Receptor (ER) Modulators, which modulate ER alpha activity, Aromatase Inhibitors (AIs) and Ovarian ablation that decrease estrogen production and FASLODEX® (Fulvestrant) that down regulates Estrogen Receptor. Aromatase Inhibitors are often prescribed due to their superiority over Tamoxifen, for postmenopausal women with Hormone Receptor positive breast tumors, in adjuvant as well as metastatic settings. Aromatase Inhibitors by themselves however, are not effective in premenopausal women, as these individuals derive their estrogen mainly from ovaries and not extragonadal tissue.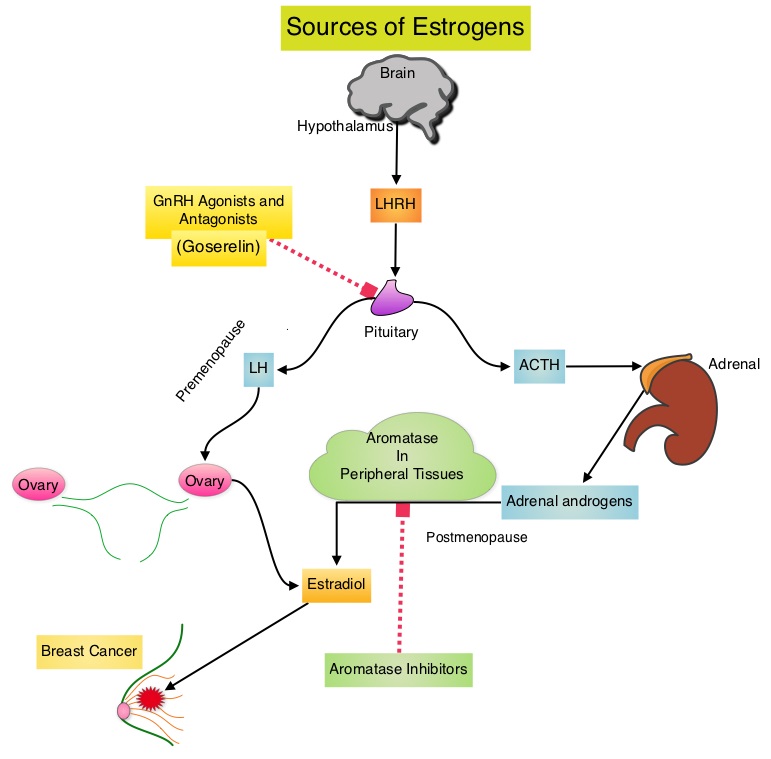
The TEXT (Tamoxifen and Exemestane Trial) and SOFT (Suppression of Ovarian Function Trial) are two phase III randomized trials, conducted at the same time and included premenopausal women (average age was 43 years) with hormone receptor positive, early breast cancer. In the joint analysis of these two trials which included 4,891 women, the authors set out to answer 2 important questions – whether adjuvant AI treatment improves outcomes in this patient group, when their Ovarian Function is suppressed and whether there is any benefit with Ovarian Function suppression in premenopausal women suitable for adjuvant Tamoxifen. TEXT randomized patients within 3 months of surgery to 5 years of AROMASIN® (Exemestane) plus Ovarian Function Suppression (OFS) or 5 years of Tamoxifen plus OFS. The SOFT study randomized patients to 5 years of AROMASIN® plus OFS or 5 years of Tamoxifen plus OFS or 5 years of Tamoxifen alone. OFS choices included oophorectomy, ovarian irradiation or 5 years of TRELSTAR® (Triptorelin), a GnRH (Gonadotropin Releasing Hormone) agonist. The 5 year Disease Free Survival was 91.1% in the AROMASIN® plus OFS group and 87.3% in the Tamoxifen plus OFS group (HR=0.72, P<0.0002). Compared to patients receiving Tamoxifen plus OFS, AROMASIN® plus OFS reduced the relative risk of premenopausal women developing a subsequent invasive breast cancer by 28% and the relative risk of breast cancer recurrence by 34%.
The authors in this analysis examined the absolute treatment effect in the TEXT and SOFT trials across a continuum of recurrence risk, to help individualize decision making for endocrine therapy, in premenopausal women with Human Epidermal growth factor Receptor 2 (HER2) -negative disease. Incorporating age, nodal status, tumor size, grade, Ki-67 expression levels and hormone receptor status, a composite recurrence risk for each patient was determined, from a Cox model.
It was noted that patients in the SOFT trial who remained premenopausal after chemotherapy experienced absolute improvement of 5% or more in 5-year Breast Cancer-Free Interval with AROMASIN® plus OFS compared with Tamoxifen plus OFS or Tamoxifen alone, and this benefit was even higher, reaching 10% to 15% for the intermediate to high composite recurrence risk group of patients. Patients in the SOFT trial whose composite recurrence risk was low did not receive chemotherapy and did well with all endocrine therapies. For patients in the TEXT trial, the benefit of AROMASIN® plus OFS compared with Tamoxifen plus OFS was similar to the SOFT trial, with the 5-year Breast Cancer-Free Interval ranging from 5-15%. Again, patients not receiving chemotherapy and with lowest composite recurrence risk did well with both endocrine therapies.
The authors concluded that premenopausal women with hormone receptor-positive, HER2-negative disease, with high risk for recurrence based on clinicopathologic features, may experience a 10% to 15% improvement in the 5-year Breast Cancer-Free Interval with AROMASIN® plus OFS compared with Tamoxifen alone. Absolute Benefit of Adjuvant Endocrine Therapies for Premenopausal Women with Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Early Breast Cancer: TEXT and SOFT Trials.Regan MM, Francis PA, Pagani O, et al. Published online before print April 4, 2016, doi: 10.1200/JCO.2015.64.3171 JCO April 4, 2016 JCO643171

