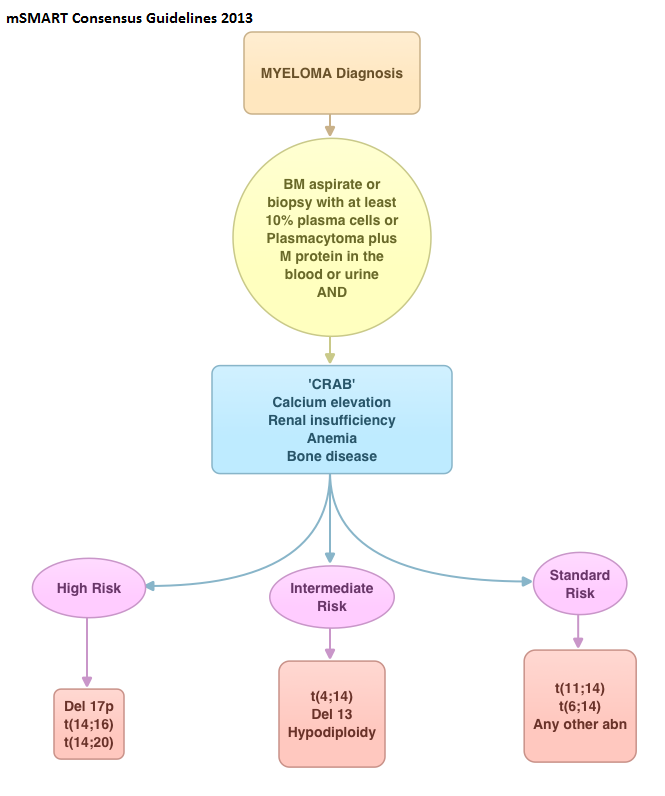
SUMMARY: In this provocative perspective, the authors suggest revising the present Multiple Myeloma (MM) International Staging System, to incorporate more relevant information related to the biology of the disease. The MM International Staging System (MM-ISS) takes into account Beta-2 Microglobulin (B2M) along with Serum Albumin (SA) levels to determine the prognosis in MM patients. 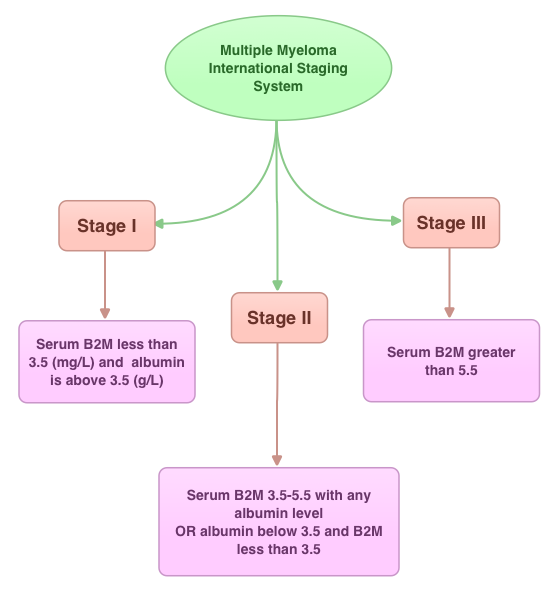 Although B2M and SA may act as surrogates for the extent of the disease, the evidence is insufficient to rely on these two entities to prognosticate MM. Even though B2M can correlate with MM tumor bulk as well as act as a biomarker of renal failure related to MM, the authors cite multiple studies suggesting that elevated serum B2M can be seen independent of MM history, in healthy elderly patients, infections, autoimmune disorders and chronic renal insufficiency. Further elevated B2M can be a marker of frailty in elderly patients and can predict disability and mortality in this patient group. With regards to Serum Albumin (SA) in MM, SA is inhibited by IL6, a MM cell growth factor and thus is able to indirectly reflect MM tumor bulk. However, low SA levels can also be seen in frail and elderly patients independent of MM history. The authors point out that the stage of the disease in the MM-ISS increases with age, independent of MM tumor bulk, due to the these nonspecific factors and does not necessarily correlate with other more specific prognostic markers such as cytogenetics, particularly in elderly patients. The authors conclude that combining the MM-ISS with tumor cytogenetics will more accurately predict prognosis in MM patients. Bataille R, Annweiler C, Beauchet O. Clinical Lymphoma Myeloma and Leukemia 2013;13:635-637
Although B2M and SA may act as surrogates for the extent of the disease, the evidence is insufficient to rely on these two entities to prognosticate MM. Even though B2M can correlate with MM tumor bulk as well as act as a biomarker of renal failure related to MM, the authors cite multiple studies suggesting that elevated serum B2M can be seen independent of MM history, in healthy elderly patients, infections, autoimmune disorders and chronic renal insufficiency. Further elevated B2M can be a marker of frailty in elderly patients and can predict disability and mortality in this patient group. With regards to Serum Albumin (SA) in MM, SA is inhibited by IL6, a MM cell growth factor and thus is able to indirectly reflect MM tumor bulk. However, low SA levels can also be seen in frail and elderly patients independent of MM history. The authors point out that the stage of the disease in the MM-ISS increases with age, independent of MM tumor bulk, due to the these nonspecific factors and does not necessarily correlate with other more specific prognostic markers such as cytogenetics, particularly in elderly patients. The authors conclude that combining the MM-ISS with tumor cytogenetics will more accurately predict prognosis in MM patients. Bataille R, Annweiler C, Beauchet O. Clinical Lymphoma Myeloma and Leukemia 2013;13:635-637