SUMMARY: Multiple Myeloma is a clonal disorder of plasma cells in the bone marrow and the American Cancer Society estimates that in the United States, about 30,330 new cases will be diagnosed in 2016 and 12,650 patients will die of the disease. Multiple Myeloma is a disease of the elderly, with a median age at diagnosis of 69 years and characterized by intrinsic clonal heterogeneity. With a record number of regulatory approvals for Myeloma treatment over the past 12 years, the median survival for patients with Myeloma is over 10 years. 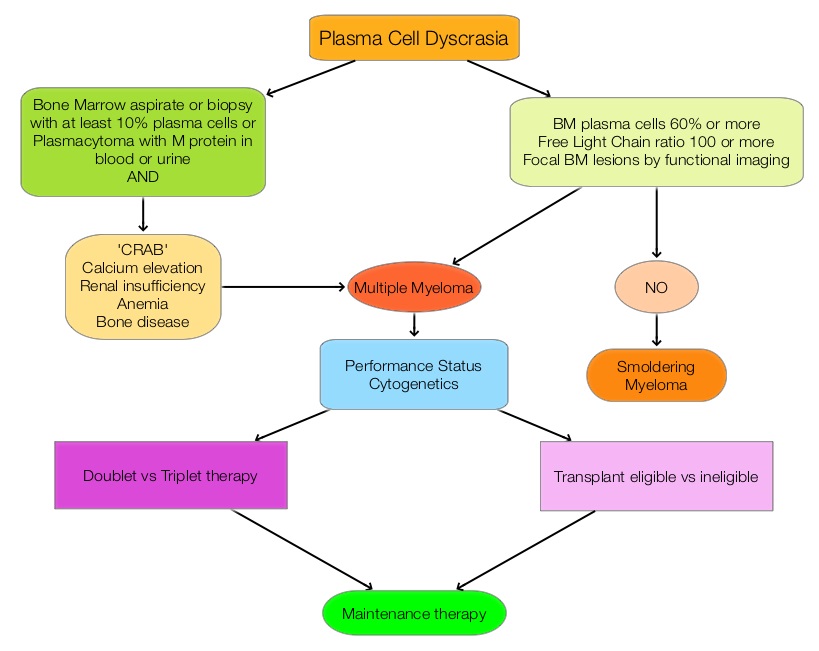 The recent new drugs approved for the treatment of relapsed/refractory Multiple Myeloma include a Histone Decetylase inhibitor (FARYDAK®) and 2 monoclonal antibodies, Daratumumab (DARZALEX®) and Elotuzumab (EMPLICITI®). The two most important determinants of Myeloma patient outcomes include, Performance Status and tumor genomics. Performance Status can be assessed using currently validated instruments and patients can be defined as Fit, Intermediate Fit, or Frail. Patients with chromosomal abnormalities t(4;14), t(14;16), t(14;20) or del 17p are considered to fall in the high risk group.
The recent new drugs approved for the treatment of relapsed/refractory Multiple Myeloma include a Histone Decetylase inhibitor (FARYDAK®) and 2 monoclonal antibodies, Daratumumab (DARZALEX®) and Elotuzumab (EMPLICITI®). The two most important determinants of Myeloma patient outcomes include, Performance Status and tumor genomics. Performance Status can be assessed using currently validated instruments and patients can be defined as Fit, Intermediate Fit, or Frail. Patients with chromosomal abnormalities t(4;14), t(14;16), t(14;20) or del 17p are considered to fall in the high risk group.
The updated Multiple Myeloma guidelines were presented at the National Comprehensive Cancer Network (NCCN) 21st Annual Conference on April 1, 2016. These guidelines include the use of the Revised International Staging System for Multiple Myeloma, treatment of asymptomatic patients even if they do not fit the CRAB criteria (Calcium elevation, Renal insufficiency, Anemia, or Bone abnormalities) and incorporation of a triplet instead of doublet for newly diagnosed Fit Myeloma patients, regardless of transplant eligibility.
Revised International Staging System for Multiple Myeloma
The revised ISS (R-ISS) combines the International Staging System (ISS) with chromosomal abnormalities detected by interphase Fluorescent In Situ Hybridization after CD138 plasma cell purification and serum Lactate DeHydrogenase (LDH).
R-ISS I: ISS stage I (serum β2-microglobulin level less than 3.5 mg/L and serum albumin level 3.5 g/dL or more), absence of high risk cytogenetics and normal LDH level (less than the upper limit of normal range)
R-ISS III: ISS stage III (serum β2-microglobulin level more than 5.5 mg/L) and high-risk cytogenetics or high LDH level
R-ISS II: Includes all the other possible combinations
New Definition of Active MM in Asymptomatic Patients Qualifying for Treatment
• Bone marrow plasmacytosis 60% or more
• Abnormal free light chain ratio 100 or more (involved kappa) or less than 0.01 (involved lambda)
• Focal bone marrow lesions detected by functional imaging such as a PET scan or a MRI
New Treatment Options
For newly diagnosed patients, who are transplant candidates or Fit non-transplant candidates, Lenalidomide/Bortezomib/Dexamethasone should be incorporated as the preferred regimen based on a phase III randomized trial showing superiority of this triplet therapy over a doublet. This triplet improved Complete Response (CR) rates (including molecular CR), Progression Free Survival (PFS), and Overall Survival (OS) compared with Lenalidomide/Dexamethasone doublet. (Blood. 2015;126:25). Another all oral triplet included in the guidelines is a combination of Lenalidomide, Ixazomib and Dexamethasone. Ixazomib (NINLARO®) is an oral proteasome inhibitor and has a half-life of 3 to 4 days and requires only once weekly administration. The combination of Carfilzomib/Lenalidomide, and Dexamethasone was included in the new guidelines as a category 2A front-line treatment, based on a phase 1/2 study (Blood. 2012;120:1801-1809). Maintenance therapy is now considered standard of care regardless of transplantation and for newly diagnosed non-transplant candidates, a new standard of care is continuous Lenalidomide/Dexamethasone, which was found to improve PFS significantly, with a favorable safety profile (N Engl J Med. 2014;371:906-917). Three drug maintenance therapy may benefit high risk patients and should be considered.
In conclusion, these latest updates reflect the rapid advances in the diagnosis and treatment of Multiple Myeloma. National Comprehensive Cancer Network (NCCN) 21st Annual Conference. Presented April 1, 2016, Hollywood, FL.

