SUMMARY: Multiple Myeloma is a clonal disorder of plasma cells in the bone marrow and the American Cancer Society estimates that in the United States, about 30,330 new cases will be diagnosed in 2016 and 12,650 patients will die of the disease. Multiple Myeloma is a disease of the elderly, with a median age at diagnosis of 69 years and characterized by intrinsic clonal heterogeneity. With a record number of regulatory approvals for Myeloma treatment over the past 12 years, the median survival for patients with Myeloma is over 10 years. The recent new drugs approved for the treatment of relapsed/refractory Multiple Myeloma include a Histone Decetylase inhibitor (FARYDAK®) and 2 monoclonal antibodies, Daratumumab (DARZALEX®) and Elotuzumab (EMPLICITI®).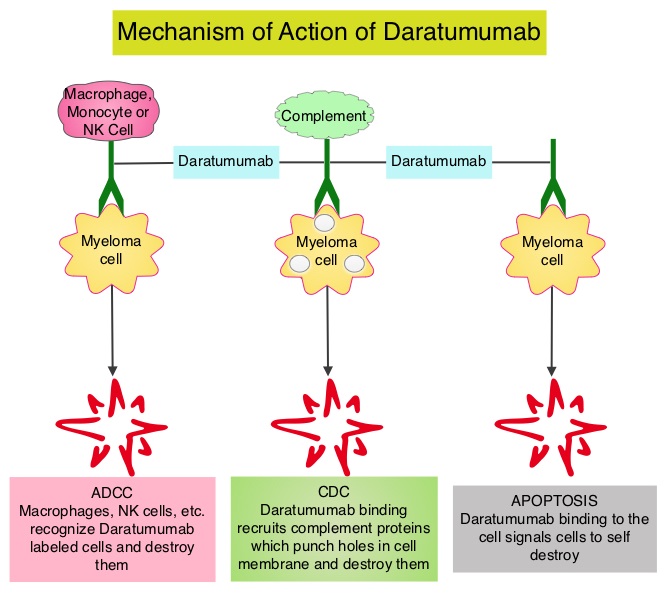
Daratumumab (DARZALEX®) is a human IgG1 antibody that targets CD38, a transmembrane glycoprotein abundantly expressed on malignant plasma cells and with low levels of expression on normal lymphoid and myeloid cells. DARZALEX® exerts its cytotoxic effect on myeloma cells by multiple mechanisms, including Antibody Dependent Cellular Cytotoxicity (ADCC), Complement Mediated Cytotoxicity and direct apoptosis. Additionally, DARZALEX® may have a role in immunomodulation by depleting CD38-positive regulator Immune suppressor cells, and thereby expanding T cells, in patients responding to therapy. Previously published phase I and II studies involving patients with relapsed or refractory multiple myeloma demonstrated promising efficacy of DARZALEX® when given as a single agent, as well as when given along with Lenalidomide (REVLIMID®) and Dexamethasone.
The authors now report the results of a prespecified interim analysis of a phase III trial of DARZALEX®, REVLIMID® and Dexamethasone in patients with relapsed or refractory multiple myeloma. In this randomized, open-label, multicenter, phase III study, 569 patients who had relapsed or refractory multiple myeloma were assigned in a 1:1 ratio to receive either DARZALEX®, REVLIMID® and Dexamethasone (Daratumumab group, N=286) or REVLIMID® and Dexamethasone (Control group, N=283). Patients refractory to REVLIMID® were excluded. Patients in the Daratumumab group received DARZALEX® 16 mg/kg IV on days 1, 8, 15, and 22 of a 28 day cycle for 8 weeks during cycles 1 and 2, every 2 weeks (on days 1 and 15) for 16 weeks (cycles 3 thru 6), and every 4 weeks thereafter. Both treatment groups received REVLIMID® 25 mg PO on days 1-21 of each cycle and Dexamethasone 40 mg PO weekly. The primary end point was Progression Free Survival (PFS). Secondary end points included the time to disease progression, Response Rate, time to response, duration of response, Overall Survival (OS) and percentage of patients with results below the threshold for Minimal Residual Disease. Minimal Residual Disease status was evaluated for patients who had a Complete Response by Next-Generation sequencing assay of bone marrow.
At a median follow-up of 13.5 months, the PFS at 12 months was 83.2% in the Daratumumab group compared to 60.1% in the control group (HR=0.37; P<0.001). The Overall Response Rate was significantly higher in the Daratumumab group than in the control group (92.9% versus 76.4%, P<0.001) and further, there was a higher rate of Complete Response or better (43.1% vs. 19.2%, P<0.001). In the Daratumumab group, 22.4% of the patients had results below the threshold for Minimal Residual Disease (1 tumor cell per 105 white cells), as compared with 4.6% of those in the control group (P<0.001), and those with results below the threshold for Minimal Residual Disease had improved outcomes. The most common grade 3 or 4 adverse events were cytopenias. Approximately 48% of the patients in the Daratumumab group experienced grade 1 or 2 infusion-related reactions.
The authors concluded that the addition of DARZALEX® to REVLIMID® and Dexamathasone significantly improves Progression Free Survival among patients with relapsed or refractory multiple myeloma. This impressive efficacy data may warrant the use of this triplet combination for first relapse, in this group of patients, provided their disease is not refractory to REVLIMID®. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. Dimopoulos MA, Oriol A, Nahi H, et al. for the POLLUX Investigators. N Engl J Med 2016;375:1319-1331

