SUMMARY: The FDA on June 16, 2017 approved the use of DARZALEX® (Daratumumab) in combination with POMALYST® (Pomalidomide) and Dexamethasone for the treatment of patients with Multiple Myeloma who have received at least two prior therapies including REVLIMID® (Lenalidomide) and a Proteasome Inhibitor. Multiple Myeloma is a clonal disorder of plasma cells in the bone marrow and the American Cancer Society estimates that in the United States, about 30,280 new cases will be diagnosed in 2017 and 12,590 patients will die of the disease. Multiple Myeloma is a disease of the elderly, with a median age at diagnosis of 69 years and characterized by intrinsic clonal heterogeneity. With a record number of regulatory approvals for Myeloma treatment over the past 12 years, the median survival for patients with Myeloma is over 10 years.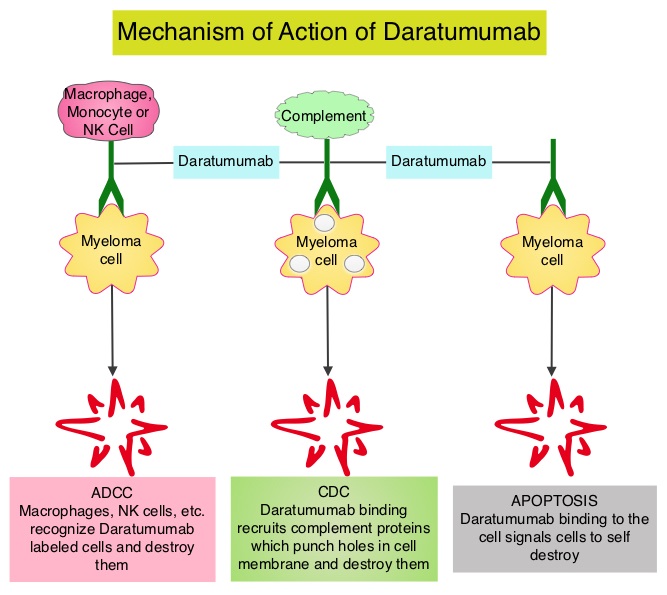
DARZALEX® is a human IgG1 antibody that targets CD38, a transmembrane glycoprotein abundantly expressed on malignant plasma cells and with low levels of expression on normal lymphoid and myeloid cells. DARZALEX® exerts its cytotoxic effect on myeloma cells by multiple mechanisms, including Antibody Dependent Cellular Cytotoxicity (ADCC), Complement Mediated Cytotoxicity and direct apoptosis. Additionally, DARZALEX® may have a role in immunomodulation by depleting CD38-positive regulator Immune suppressor cells, and thereby expanding T cells, in patients responding to therapy. The FDA approved DARZALEX® in November 2015 as monotherapy for Myeloma patients who had received at least three prior lines of therapy including a Proteasome Inhibitor (PI) and an Immunomodulatory agent or who are double refractory to a PI and an Immunomodulatory agent. In November 2016, DARZALEX® was approved in combination with REVLIMID® and Dexamethasone, or VELCADE® (Bortezomib) and Dexamethasone, for the treatment of patients with Multiple Myeloma who have received at least one prior therapy. POMALYST® (Pomalidomide) is a novel, oral, immunomodulatory drug which is far more potent than THALOMID® (Thalidomide) and REVLIMID®, and has been shown to be active in REVLIMID® and VELCADE® refractory patients.
This new FDA approval was based on data from the phase Ib (MMY1001, EQUULEUS) study of DARZALEX® in combination with POMALYST® and Dexamethasone in relapsed or refractory Multiple Myeloma. This open-label study included 103 patients with Multiple Myeloma who had received prior treatment with a Proteasome Inhibitor and an Immunomodulatory agent. Treatment consisted of DARZALEX® 16 mg/kg IV on days 1, 8, 15, and 22 of a 28 day cycle for 8 weeks during cycles 1 and 2, every 2 weeks (on days 1 and 15) for 16 weeks (cycles 3 thru 6), and every 4 weeks thereafter until disease progression. POMALYST® 4 mg PO was administered daily for 21 days along with Dexamethasone 40 mg weekly (20 mg for patients over 75 years of age). The median patient age was 64 years and patients had received a median of 4 prior lines of therapy. About 75% of the patients had prior Autologous Stem Cell Transplant, 90% of patients were refractory to REVLIMID®, 70% were refractory to VELCADE®, and 64% were refractory to both agents.
The Overall Response Rate in this study was 59% with Very Good Partial Response (VGPR) noted in 28% of patients. Complete Response was achieved in 6% of patients and stringent Complete Response was achieved in 8% of patients. The median time to response was 1 month and the median duration of response was 13.6 months. The most common toxicities were infusion reactions, nausea, vomiting, diarrhea, fatigue, fever, upper respiratory tract infection, muscle spasms, cough and dyspnea. The most common grade 3/4 toxicities were cytopenias including lymphopenia.
It was concluded that DARZALEX® in combination with POMALYST® and Dexamethasone is a new combination therapy, with significant clinical benefit, for patients who relapse or become resistant to Proteasome Inhibitors and Immunomodulatory agents. This combination may be a viable option for patients who progress on a combination of REVLIMID®, VELCADE® and Dexamethasone (RVD) regimen, which is often given as first line therapy. A Study of JNJ-54767414 (HuMax CD38) (Anti-CD38 Monoclonal Antibody) in Combination With Backbone Treatments for the Treatment of Patients With Multiple Myeloma. ClinicalTrials.gov Identifier: NCT01998971 https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/761036orig1s005ltr.pdf.

