SUMMARY: The American Cancer Society estimates that approximately 15,720 new cases of chronic lymphocytic leukemia (CLL) will be diagnosed in 2014 and approximately 4600 patients will die from the disease. CLL is a disease of the elderly and the average age at the time of diagnosis is 72 years. There are two main types of lymphocytes, B and T lymphocytes/cells, and B-cell CLL is the most common type of leukemia in adults. Normal B-cell activation and proliferation is dependent on B-cell receptor (BCR) signaling.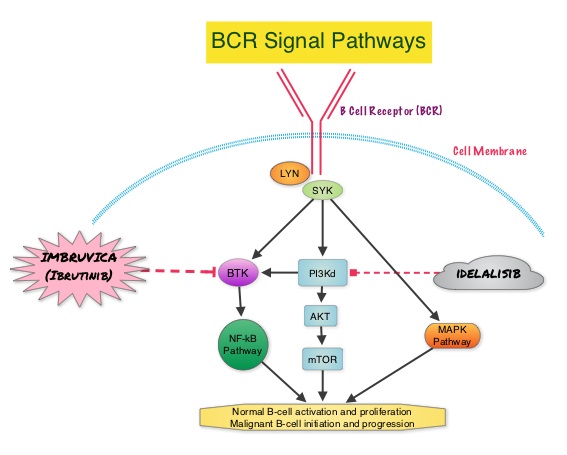 This signaling is also important for initiation and progression of B-cell lymphoproliferative disorders. Bruton’s tyrosine kinase (BTK) is a member of the Tec family of kinases, downstream of the B-cell receptor and is predominantly expressed in B-cells. It is a mediator of B-cell receptor signaling in normal and transformed B-cells. Following binding of antigen to the BCR, Syk (Spleen Tyrosine Kinase), Lyn (member of the Src family of protein tyrosine kinases) and BTK (Bruton’s Tyrosine Kinase) are activated, with subsequent propagation through PI3K/Akt, MAPK, and NF-κB pathways and resulting B-cell activation and proliferation. IMBRUVICA® (Ibrutinib) is an oral, irreversible inhibitor of BTK and inhibits cell proliferation and promotes programmed cell death (Apoptosis). The RESONATE trial is a multicenter, randomized, open-label Phase III study in which single agent IMBRUVICA® was compared to single agent ARZERRA® (Ofatumumab) in patients with relapsed or refractory CLL or Small Lymphocytic Lymphoma (SLL). In this study, 391 patients who had measurable nodal disease and received at least one prior therapy, were randomized to receive 420 mg of IMBRUVICA® orally once daily until progression (N=195) or ARZERRA® at an initial dose of 300 mg followed by 11 doses at 2000 mg, given intravenously weekly (N=196). Patients randomized to the ARZERRA® group, on disease progression were allowed to receive treatment with IMBRUVICA®. The median age was 67 years, 40% of the patients enrolled in the study were 70 years of age or over and 30% of patients had deletion of chromosome 17p. The primary endpoint of this study was Progression-Free Survival (PFS) and the secondary endpoints included Overall Survival (OS), Overall Response rate (ORR) and safety. Following recommendations from the Independent Data Monitoring Committee (IDMC), the study was stopped earlier, as the primary endpoint as well as an important secondary endpoint of the study, were met. At a median follow up of 9.4 months, IMBRUVICA® significantly prolonged PFS compared to ARZERRA® (median not reached vs 8.1 months; HR 0.215, P<0.0001) with a 78.5% reduction in the risk of disease progression and also significantly improved OS (median not reached, HR 0.43, P=0.0049) when compared with ARZERRA®, with a 57% reduction in the risk of death. The Overall Response Rates were significantly higher in the IMBRUVICA® group compared to the ARZERRA® group (42.6% vs 4.1% (P <0 .0001). An additional 20% of patients treated with IMBRUVICA® had a partial response of their persistent lymphocytosis. The benefit with IMBRUVICA® was similarly high even in the two very high risk groups of patients such as those with 17p deletions and those refractory to purine analog chemoimmunotherapy. The overall survival was significant despite the crossover of 57 patients upon progression, from the ARZERRA® group to IMBRUVICA®. Treatment was well tolerated in both groups. Diarrhea, fatigue, nausea and atrial fibrillation were more frequent in the IMBRUVICA® group but did not result in frequent dose reductions or treatment discontinuation. The authors concluded that IMBRUVICA® significantly improved Progression Free Survival, Overall Survival and Overall Response Rates, in patients with relapsed/refractory CLL/SLL, compared with ARZERRA® and IMBRUVICA® should also be a consideration for elderly patients who often are unable to tolerate intensive chemotherapy. Byrd JC, Brown JR, O’Brien SM, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr LBA7008)
This signaling is also important for initiation and progression of B-cell lymphoproliferative disorders. Bruton’s tyrosine kinase (BTK) is a member of the Tec family of kinases, downstream of the B-cell receptor and is predominantly expressed in B-cells. It is a mediator of B-cell receptor signaling in normal and transformed B-cells. Following binding of antigen to the BCR, Syk (Spleen Tyrosine Kinase), Lyn (member of the Src family of protein tyrosine kinases) and BTK (Bruton’s Tyrosine Kinase) are activated, with subsequent propagation through PI3K/Akt, MAPK, and NF-κB pathways and resulting B-cell activation and proliferation. IMBRUVICA® (Ibrutinib) is an oral, irreversible inhibitor of BTK and inhibits cell proliferation and promotes programmed cell death (Apoptosis). The RESONATE trial is a multicenter, randomized, open-label Phase III study in which single agent IMBRUVICA® was compared to single agent ARZERRA® (Ofatumumab) in patients with relapsed or refractory CLL or Small Lymphocytic Lymphoma (SLL). In this study, 391 patients who had measurable nodal disease and received at least one prior therapy, were randomized to receive 420 mg of IMBRUVICA® orally once daily until progression (N=195) or ARZERRA® at an initial dose of 300 mg followed by 11 doses at 2000 mg, given intravenously weekly (N=196). Patients randomized to the ARZERRA® group, on disease progression were allowed to receive treatment with IMBRUVICA®. The median age was 67 years, 40% of the patients enrolled in the study were 70 years of age or over and 30% of patients had deletion of chromosome 17p. The primary endpoint of this study was Progression-Free Survival (PFS) and the secondary endpoints included Overall Survival (OS), Overall Response rate (ORR) and safety. Following recommendations from the Independent Data Monitoring Committee (IDMC), the study was stopped earlier, as the primary endpoint as well as an important secondary endpoint of the study, were met. At a median follow up of 9.4 months, IMBRUVICA® significantly prolonged PFS compared to ARZERRA® (median not reached vs 8.1 months; HR 0.215, P<0.0001) with a 78.5% reduction in the risk of disease progression and also significantly improved OS (median not reached, HR 0.43, P=0.0049) when compared with ARZERRA®, with a 57% reduction in the risk of death. The Overall Response Rates were significantly higher in the IMBRUVICA® group compared to the ARZERRA® group (42.6% vs 4.1% (P <0 .0001). An additional 20% of patients treated with IMBRUVICA® had a partial response of their persistent lymphocytosis. The benefit with IMBRUVICA® was similarly high even in the two very high risk groups of patients such as those with 17p deletions and those refractory to purine analog chemoimmunotherapy. The overall survival was significant despite the crossover of 57 patients upon progression, from the ARZERRA® group to IMBRUVICA®. Treatment was well tolerated in both groups. Diarrhea, fatigue, nausea and atrial fibrillation were more frequent in the IMBRUVICA® group but did not result in frequent dose reductions or treatment discontinuation. The authors concluded that IMBRUVICA® significantly improved Progression Free Survival, Overall Survival and Overall Response Rates, in patients with relapsed/refractory CLL/SLL, compared with ARZERRA® and IMBRUVICA® should also be a consideration for elderly patients who often are unable to tolerate intensive chemotherapy. Byrd JC, Brown JR, O’Brien SM, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr LBA7008)
Tag: Chronic Lymphocytic Leukemia
CD49d Is the Strongest Flow Cytometry–Based Predictor of Overall Survival in Chronic Lymphocytic Leukemia
SUMMARY:The American Cancer Society's estimates that approximately 15,720 new cases of chronic lymphocytic leukemia (CLL) will be diagnosed in 2014 and approximately 4600 patients will die from the disease. CLL is a heterogeneous disease with a clinical course that is variable, with a very indolent course in some patients and some with aggressive disease and others somewhere in between. Both Binet and the Rai CLL staging systems developed in the 1970’s rely solely on physical examination and standard laboratory testing to predict survival. With the development of Interphase Fluorescent In Situ Hybridization (FISH) technique, which allows detection of genetic abnormalities in noncycling CLL cells, it has become clear that cytogenetic abnormalities are often seen in CLL patients and these genetic abnormalities in turn appear to be reliable predictors of disease progression, response to therapy and survival. Some of these cytogenetic abnormalities include del(13q), normal karyotype, trisomy(12), del(11q), del(17p), and they are associated with decreasing survival times, in that order. Another important prognostic factor is the rearrangement and somatic hypermutation of the variable region of the immunoglobulin heavy chain genes (IGHV), which is an independent predictor of outcome in CLL.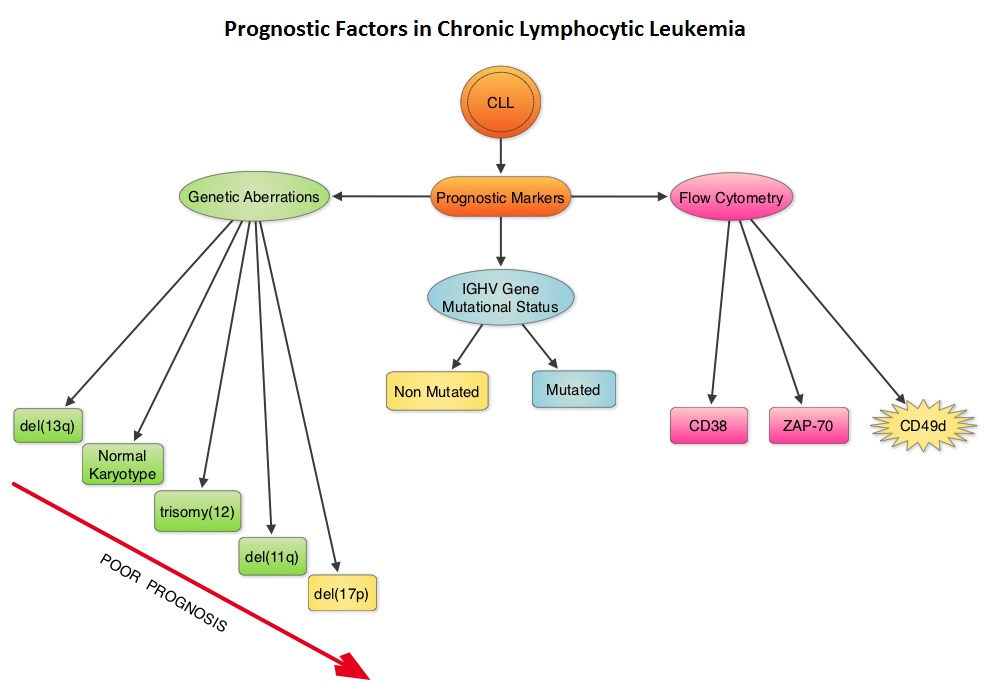 Retrospective studies have suggested that patients with CLL whose leukemic cells have clonotypically rearranged immunoglobulin genes in germline configuration (Unmutated IGHV gene) demonstrated more aggressive disease and shorter survival time compared to those patients with somatic hypermutations in their IGHV genes (Mutated IGHV gene). Expression of two flow cytometry based biomarkers, CD38 (surface marker) and ZAP-70 (intracytoplasmic protein), have been associated with poor outcomes. As we learn more about the pathobiology of CLL, it is becoming clear that survival of CLL cells is dependent not only on their intracellular defects but also on the microenvironment. CD49d, an integrin, is a surface molecule, detected by flow cytometry. CD49d expression promotes microenvironment mediated proliferation of CLL cells and has been associated with shortened survival. The authors conducted this multicenter analysis to evaluate the prognostic utility of CD49d in CLL, in comparison with CD38 and ZAP-70. The authors analysed the data of 2972 patients from 9 clinical trials. All these studies had data on CD49d expression of CLL cells by flow cytometry and reported the association between CD49d expression and Overall survival and/or Treatment Free Survival and/or Progression Free Survival. Patients with 30% or more of CLL cells expressing CD49d were considered CD49d positive. In this pooled analysis, CD49d positive patients had a significantly lower Overall Survival both at 5 years (87% vs 94%) and 10 years (62% vs 84%) compared with CD49d negative patients (P<0.001). Further, CD49d positive patients more likely required treatment, suggesting that these patients had a lower probability of remaining treatment free at both 5 years (42% vs 68%) and 10 years (24% vs 50%), compared with CD49d negative patients. When other variables were taken into consideration, CD49d was the only flow cytometry based marker which independently predicted Overall Survival with greater prognostic relevance than CD38 and ZAP-70. The authors concluded that CD49d expression and IGHV gene mutational status may be the strongest predictors of Overall Survival and Treatment Free Survival in patients with CLL and should be a part of routine baseline testing at the time of diagnosis. Bulian P, Shanafelt TD, Fegan C, et al. J Clin Oncol 2014;32:897-904
Retrospective studies have suggested that patients with CLL whose leukemic cells have clonotypically rearranged immunoglobulin genes in germline configuration (Unmutated IGHV gene) demonstrated more aggressive disease and shorter survival time compared to those patients with somatic hypermutations in their IGHV genes (Mutated IGHV gene). Expression of two flow cytometry based biomarkers, CD38 (surface marker) and ZAP-70 (intracytoplasmic protein), have been associated with poor outcomes. As we learn more about the pathobiology of CLL, it is becoming clear that survival of CLL cells is dependent not only on their intracellular defects but also on the microenvironment. CD49d, an integrin, is a surface molecule, detected by flow cytometry. CD49d expression promotes microenvironment mediated proliferation of CLL cells and has been associated with shortened survival. The authors conducted this multicenter analysis to evaluate the prognostic utility of CD49d in CLL, in comparison with CD38 and ZAP-70. The authors analysed the data of 2972 patients from 9 clinical trials. All these studies had data on CD49d expression of CLL cells by flow cytometry and reported the association between CD49d expression and Overall survival and/or Treatment Free Survival and/or Progression Free Survival. Patients with 30% or more of CLL cells expressing CD49d were considered CD49d positive. In this pooled analysis, CD49d positive patients had a significantly lower Overall Survival both at 5 years (87% vs 94%) and 10 years (62% vs 84%) compared with CD49d negative patients (P<0.001). Further, CD49d positive patients more likely required treatment, suggesting that these patients had a lower probability of remaining treatment free at both 5 years (42% vs 68%) and 10 years (24% vs 50%), compared with CD49d negative patients. When other variables were taken into consideration, CD49d was the only flow cytometry based marker which independently predicted Overall Survival with greater prognostic relevance than CD38 and ZAP-70. The authors concluded that CD49d expression and IGHV gene mutational status may be the strongest predictors of Overall Survival and Treatment Free Survival in patients with CLL and should be a part of routine baseline testing at the time of diagnosis. Bulian P, Shanafelt TD, Fegan C, et al. J Clin Oncol 2014;32:897-904
ARZERRA® combination for Frontline Treatment of CLL
The FDA on April 17, 2014 approved ARZERRA® (Ofatumumab) in combination with LEUKERAN® (Chlorambucil), for the treatment of previously untreated patients with Chronic Lymphocytic Leukemia (CLL), for whom FLUDARA® (Fludarabine) based therapy is considered inappropriate. ARZERRA® is a second generation fully human IgG 1 monoclonal antibody and unlike RITUXAN® (Rituximab) which is a chimeric monoclonal antibody, targets a different region (different epitope) of the CD20 molecule. The combination of ARZERRA® given along with LEUKERAN® significantly improved Progression Free Survival, Response Rates and duration of response, compared to single agent LEUKERAN®. ARZERRA® in combination with LEUKERAN® is a clinically important milestone, in the management of elderly patients with CLL.
Ofatumumab + Chlorambucil versus Chlorambucil alone in Patients with Untreated Chronic Lymphocytic Leukemia (CLL) Results of the Phase III Study Complement 1 (OMB110911)
SUMMARY: The American Cancer Society's estimates that approximately 15,720 new cases of chronic lymphocytic leukemia (CLL) will be diagnosed in 2014 and approximately 4600 patients will die from the disease. CLL is a disease of the elderly and the average age at the time of diagnosis is 72 years. Majority of these patients have associated comorbidities and would be considered inappropriate for Fludarabine (FLUDARA®) based therapy. COMPLEMENT 1 is a randomized, open-label, multicenter, phase III trial in which Ofatumumab (ARZERRA®) in combination with Chlorambucil (LEUKERAN®) was compared to single agent LEUKERAN®. ARZERRA® is a second generation fully human IgG 1 monoclonal antibody. Unlike Rituximab (RITUXAN®) which is the chimeric monoclonal antibody, ARZERRA® targets a different region (different epitope) of the CD20 molecule. To go back to basics, several antigen molecules are expressed on the surface of normal B cells. Majority of these antigens are involved in cell growth, proliferation, differentiation, immune regulation and complement activation. The various stages of B cell development include hematopoietic stem cell, lymphoid stem cell, Pro B cell, Pre B cell, Immature B cell and Mature B-cell, Activated B cell, Memory B cell and Plasma cell. The CD20 molecule is expressed at specific stages of B cell development (Pre B cell stage to Mature B lymphocyte stage) and on malignant B cells. This molecule however is not expressed on hematopoietic stem cells and plasma cells. As such, targeting CD20 with therapeutic monoclonal antibodies spares the Pro B cell which is a precursor of Pre B cell and this along with intact hematopoietic stem cell facilitates post treatment recovery of B cells. As the plasma cells are spared as well, serum IgG levels are maintained. Monoclonal antibodies targeting CD20 destroy CD20 positive B cells by 3 different mechanisms. They include Antibody Dependent Cellular Cytotoxicity (ADCC), Complement Dependent Cytotoxicity (CDC) and programmed cell death (Apoptosis).
Monoclonal antibodies targeting CD20 destroy CD20 positive B cells by 3 different mechanisms. They include Antibody Dependent Cellular Cytotoxicity (ADCC), Complement Dependent Cytotoxicity (CDC) and programmed cell death (Apoptosis).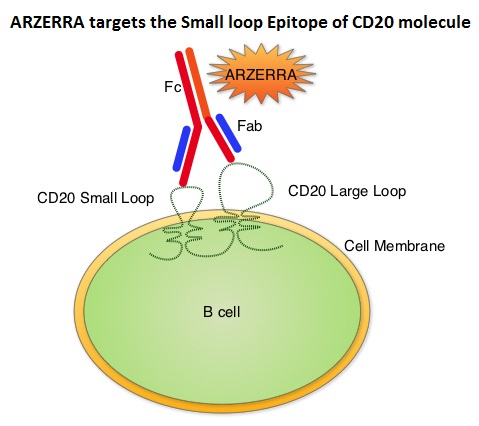 Unlike RITUXAN®, ARZERRA® targets the Small loop epitope of CD20 molecule which is proximal to the B cell membrane and this has been shown to be associated with highly efficient cell lysis through complement dependent cytotoxicity. So, compared to RITUXAN®. ARZERRA® has stronger CDC, similar ADCC and does not appear to induce Apoptosis. In this study, 447 CLL patients for whom FLUDARA® based therapy was considered to be inappropriate due to age and comorbidities, were randomly assigned 1:1 to receive either ARZERRA® in combination with LEUKERAN® or LEUKERAN® alone. ARZERRA® was given as an IV infusion at a dose of 300 mg on Cycle 1, Day 1, 1000 mg on Cycle 1, Day 8 and 1000 mg administered on Day 1 of all subsequent 28 day cycles. LEUKERAN® was given at a dose of 10 mg/m2 orally on Days 1 to 7 every 28 days in both treatment groups. The median age was 69 years and majority of the patients had 2 or more comorbidities. The primary endpoint of this study was Progression Free Survival (PFS) and secondary endpoints included Overall Response Rate (ORR), Overall Survival (OS) and safety. The median number of cycles in both treatment groups was 6. The median PFS was 22.4 months for patients receiving ARZERRA® in combination with LEUKERAN® compared with 13.1 months for those receiving single agent LEUKERAN® (HR=0.57, P< 0.001). The ORR was higher with the combination regimen versus single agent LEUKERAN® (82% vs 69%, P=0.001) and 37% of patients in the combination arm were MRD negative. The median OS for the combination group was not reached. The majority of adverse reactions were Grade 2 or lower, in both of the treatment arms and included infusion reactions, neutropenia, asthenia, headache, herpes simplex, lower respiratory tract infections, arthralgia and upper abdominal pain. The authors concluded that ARZERRA® in combination with LEUKERAN® is a clinically important milestone, in the management of elderly patients with CLL, who are considered inappropriate for FLUDARA® based therapy. Hillmen P, Robak T, Janssens A, et al. Blood 2013;122: Abstract#528
Unlike RITUXAN®, ARZERRA® targets the Small loop epitope of CD20 molecule which is proximal to the B cell membrane and this has been shown to be associated with highly efficient cell lysis through complement dependent cytotoxicity. So, compared to RITUXAN®. ARZERRA® has stronger CDC, similar ADCC and does not appear to induce Apoptosis. In this study, 447 CLL patients for whom FLUDARA® based therapy was considered to be inappropriate due to age and comorbidities, were randomly assigned 1:1 to receive either ARZERRA® in combination with LEUKERAN® or LEUKERAN® alone. ARZERRA® was given as an IV infusion at a dose of 300 mg on Cycle 1, Day 1, 1000 mg on Cycle 1, Day 8 and 1000 mg administered on Day 1 of all subsequent 28 day cycles. LEUKERAN® was given at a dose of 10 mg/m2 orally on Days 1 to 7 every 28 days in both treatment groups. The median age was 69 years and majority of the patients had 2 or more comorbidities. The primary endpoint of this study was Progression Free Survival (PFS) and secondary endpoints included Overall Response Rate (ORR), Overall Survival (OS) and safety. The median number of cycles in both treatment groups was 6. The median PFS was 22.4 months for patients receiving ARZERRA® in combination with LEUKERAN® compared with 13.1 months for those receiving single agent LEUKERAN® (HR=0.57, P< 0.001). The ORR was higher with the combination regimen versus single agent LEUKERAN® (82% vs 69%, P=0.001) and 37% of patients in the combination arm were MRD negative. The median OS for the combination group was not reached. The majority of adverse reactions were Grade 2 or lower, in both of the treatment arms and included infusion reactions, neutropenia, asthenia, headache, herpes simplex, lower respiratory tract infections, arthralgia and upper abdominal pain. The authors concluded that ARZERRA® in combination with LEUKERAN® is a clinically important milestone, in the management of elderly patients with CLL, who are considered inappropriate for FLUDARA® based therapy. Hillmen P, Robak T, Janssens A, et al. Blood 2013;122: Abstract#528
ARZERRA® (Ofatumumab)
ARZERRA® (Ofatumumab): The FDA on April 17, 2014 approved ARZERRA® in combination with LEUKERAN® (Chlorambucil), for the treatment of previously untreated patients with Chronic Lymphocytic Leukemia (CLL), for whom FLUDARA® (Fludarabine) based therapy is considered inappropriate. ARZERRA® first received accelerated approval in 2009, for the treatment of patients with CLL, refractory to FLUDARA® and CAMPATH® (Alemtuzumab). ARZERRA® injection is given as an intravenous infusion and is a product GlaxoSmithKline.
Obinutuzumab plus Chlorambucil in Patients with CLL and Coexisting Conditions
SUMMARY: Chronic Lymphocytic leukemia (CLL) is a disease of the elderly with a median age at diagnosis of 72 years. Given the age at diagnosis, it is not uncommon for these patients to have multiple comorbidities. The authors in this trial attempted to study a new agent, Obinutuzumab or GAZYVA® (GA101) specifically in this patient population.  GAZYVA® is glycoengineered, fully humanized, third generation, type II anti-CD20 antibody (IgG1 monoclonal antibody) that selectivity binds to the extracellular domain of the CD20 antigen on malignant human B cells. By virtue of binding affinity of the glycoengineered Fc portion of GAZYVA® to Fcγ receptor III on innate immune effector cells such as natural killer cells, macrophages and neutrophils, antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis is significantly enhanced, whereas it induces very little complement-dependent cytotoxicity. This is in contrast to RITUXAN® (Rituximab), which is a first generation type I, chimeric anti-CD20 targeted monoclonal antibody that kills CLL cells primarily by complement-dependent cytotoxicity and also ADCC. In this phase III trial, LEUKERAN® (Chlorambucil) was compared with a combination of GAZYVA® plus LEUKERAN® and a combination of RITUXAN® plus LEUKERAN®. Five Hundred and eighty nine (589) treatment naïve CLL patients over 70 years of age with comorbidities were enrolled of whom 118 patients received LEUKERAN® alone, 238 received GAZYVA® plus LEUKERAN® and 233 received RITUXAN® plus LEUKERAN®. The primary endpoint was Progression-Free Survival (PFS). Chemoimmunotherapy with both GAZYVA® plus LEUKERAN® and RITUXAN® plus LEUKERAN® significantly prolonged PFS compared to LEUKERAN® alone. The median PFS was 11.1 months with LEUKERAN® alone compared to 26.7 months for GAZYVA® plus LEUKERAN® (HR=0.18, P<0.001) and 16.3 months for RITUXAN® plus LEUKERAN® (HR=0.44, P<0.001). This benefit was seen in all subgroups except those with del(17) and quality of life in those who received antibody along with LEUKERAN® was not compromised. The combination of GAZYVA® and LEUKERAN®, also prolonged overall survival when compared to LEUKERAN® alone (HR=0.41; P=0.002). This benefit however was not noted with the RITUXAN® plus LEUKERAN® combination. Treatment with GAZYVA® plus LEUKERAN® when compared with RITUXAN® plus LEUKERAN®, resulted in a longer PFS (26.7 vs15.2 months; HR=0.39; P<0.001), higher complete response rates (20.7% vs. 7.0%) and deeper molecular responses. Infusion related reactions were more common in the GAZYVA® plus LEUKERAN® group without increase in the risk for infections. The authors concluded that a combination of GAZYVA® and LEUKERAN® when given to elderly patients with comorbid conditions improved overall survival compared to LEUKERAN® alone and resulted in higher response rates and longer PFS than RITUXAN® plus LEUKERAN®. Goede V, Fischer K, Busch R, et al. N Engl J Med 2014; 370:1101-1110
GAZYVA® is glycoengineered, fully humanized, third generation, type II anti-CD20 antibody (IgG1 monoclonal antibody) that selectivity binds to the extracellular domain of the CD20 antigen on malignant human B cells. By virtue of binding affinity of the glycoengineered Fc portion of GAZYVA® to Fcγ receptor III on innate immune effector cells such as natural killer cells, macrophages and neutrophils, antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis is significantly enhanced, whereas it induces very little complement-dependent cytotoxicity. This is in contrast to RITUXAN® (Rituximab), which is a first generation type I, chimeric anti-CD20 targeted monoclonal antibody that kills CLL cells primarily by complement-dependent cytotoxicity and also ADCC. In this phase III trial, LEUKERAN® (Chlorambucil) was compared with a combination of GAZYVA® plus LEUKERAN® and a combination of RITUXAN® plus LEUKERAN®. Five Hundred and eighty nine (589) treatment naïve CLL patients over 70 years of age with comorbidities were enrolled of whom 118 patients received LEUKERAN® alone, 238 received GAZYVA® plus LEUKERAN® and 233 received RITUXAN® plus LEUKERAN®. The primary endpoint was Progression-Free Survival (PFS). Chemoimmunotherapy with both GAZYVA® plus LEUKERAN® and RITUXAN® plus LEUKERAN® significantly prolonged PFS compared to LEUKERAN® alone. The median PFS was 11.1 months with LEUKERAN® alone compared to 26.7 months for GAZYVA® plus LEUKERAN® (HR=0.18, P<0.001) and 16.3 months for RITUXAN® plus LEUKERAN® (HR=0.44, P<0.001). This benefit was seen in all subgroups except those with del(17) and quality of life in those who received antibody along with LEUKERAN® was not compromised. The combination of GAZYVA® and LEUKERAN®, also prolonged overall survival when compared to LEUKERAN® alone (HR=0.41; P=0.002). This benefit however was not noted with the RITUXAN® plus LEUKERAN® combination. Treatment with GAZYVA® plus LEUKERAN® when compared with RITUXAN® plus LEUKERAN®, resulted in a longer PFS (26.7 vs15.2 months; HR=0.39; P<0.001), higher complete response rates (20.7% vs. 7.0%) and deeper molecular responses. Infusion related reactions were more common in the GAZYVA® plus LEUKERAN® group without increase in the risk for infections. The authors concluded that a combination of GAZYVA® and LEUKERAN® when given to elderly patients with comorbid conditions improved overall survival compared to LEUKERAN® alone and resulted in higher response rates and longer PFS than RITUXAN® plus LEUKERAN®. Goede V, Fischer K, Busch R, et al. N Engl J Med 2014; 370:1101-1110
Targeting BTK with Ibrutinib in Relapsed Chronic Lymphocytic Leukemia
SUMMARY: Normal B-cell activation and proliferation is dependent on B-cell receptor (BCR) signaling. This signaling is also important for initiation and progression of B-cell lymphoproliferative disorders. Bruton’s tyrosine kinase (BTK) is a member of the Tec family of kinases, downstream of the B-cell receptor and is predominantly expressed in B-cells. It is a mediator of B-cell receptor signaling in normal and transformed B-cells. Following binding of antigen to the BCR, Syk (Spleen Tyrosine Kinase), Lyn (member of the Src family of protein tyrosine kinases) and BTK (Bruton’s Tyrosine Kinase) are activated, with subsequent propagation through PI3K/Akt, MAPK, NF-κB pathways and resulting B-cell activation and proliferation. IMBRUVICA® (Ibrutinib, PCI-32765) is an oral, irreversible inhibitor of BTK and inhibits cell proliferation and promotes programmed cell death (Apoptosis).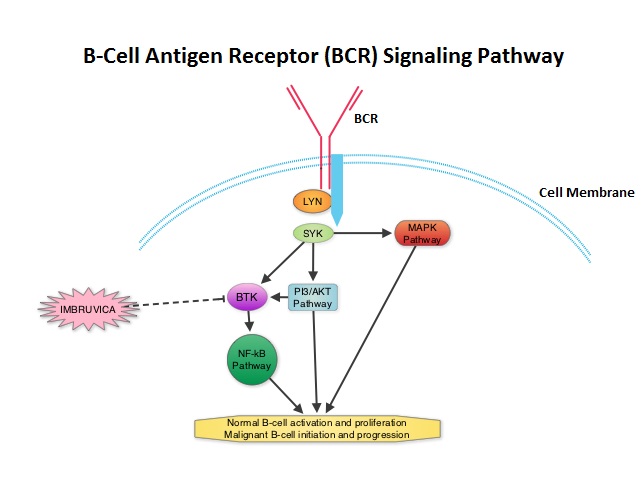 The FDA granted accelerated approval of IMBRUVICA® for the treatment of patients with Chronic Lymphocytic Leukemia (CLL) who had received at least one prior therapy. This approval was based on the outcomes in a select group of 48 patients who were a part of a larger group of 85 patients, enrolled in a multicenter single arm phase Ib/II trial. The median age was 67 years and 71% were male. Patients had a median number of 4 prior treatments and had an ECOG PS of 0-1. Patients in this group received IMBRUVICA® 420 mg PO daily until disease progression or unacceptable toxicity. The overall response rate was 58.3% as assessed by an independent review committee. No complete responses were seen and the response duration ranged from 5.6 to over 24 months. This analysis did not include data from those patients enrolled in the trial who received IMBRUVICA® 840 mg PO daily or those with Small Lymphocytic Lymphoma (N=37). The most common toxicities included fatigue, myalgias and arthralgias, cytopenias, nausea, diarrhea, fever and rash. Transient asymptomatic increase in lymphocyte count with resolution of lymphadenopathy and splenomegaly was common but resolved with continued treatment. The confirmatory RESONATE trial is a multicenter, randomized, open-label Phase III study in which single agent IMBRUVICA® was compared to single agent ARZERRA® (Ofatumumab) in patients with relapsed or refractory CLL or Small Lymphocytic Lymphoma . This was a part of the requirement by the FDA. Enrolled patients had measurable nodal disease and were not eligible for treatment with purine analog-based therapy. In this study, 391 patients who had received at least one prior therapy, were enrolled and randomized to receive 420 mg of IMBRUVICA® orally once daily or ARZERRA® given intravenously. Treatment was given over a period of 24 weeks until disease progression or unacceptable toxicity. Patients randomized to the ARZERRA® group on disease progression were allowed to receive treatment with IMBRUVICA®. The primary endpoint of this study was progression-free survival and the secondary endpoint was overall survival. Following recommendations from the Independent Data Monitoring Committee (IDMC), the study was stopped earlier, as the primary endpoint as well as an important secondary endpoint of the study were met. At the planned interim analysis, patients in the IMBRUVICA® group showed a statistically significant improvement in progression-free survival, the primary endpoint of the study as well as a statistically significant improvement in overall survival, the secondary endpoint of the trial. This data confirmed the efficacy of IMBRUVICA® and gives patients with CLL, an important new treatment option. Byrd JC, Furman RR, Coutre SE, et al. N Engl J Med 2013; 369:32-42
The FDA granted accelerated approval of IMBRUVICA® for the treatment of patients with Chronic Lymphocytic Leukemia (CLL) who had received at least one prior therapy. This approval was based on the outcomes in a select group of 48 patients who were a part of a larger group of 85 patients, enrolled in a multicenter single arm phase Ib/II trial. The median age was 67 years and 71% were male. Patients had a median number of 4 prior treatments and had an ECOG PS of 0-1. Patients in this group received IMBRUVICA® 420 mg PO daily until disease progression or unacceptable toxicity. The overall response rate was 58.3% as assessed by an independent review committee. No complete responses were seen and the response duration ranged from 5.6 to over 24 months. This analysis did not include data from those patients enrolled in the trial who received IMBRUVICA® 840 mg PO daily or those with Small Lymphocytic Lymphoma (N=37). The most common toxicities included fatigue, myalgias and arthralgias, cytopenias, nausea, diarrhea, fever and rash. Transient asymptomatic increase in lymphocyte count with resolution of lymphadenopathy and splenomegaly was common but resolved with continued treatment. The confirmatory RESONATE trial is a multicenter, randomized, open-label Phase III study in which single agent IMBRUVICA® was compared to single agent ARZERRA® (Ofatumumab) in patients with relapsed or refractory CLL or Small Lymphocytic Lymphoma . This was a part of the requirement by the FDA. Enrolled patients had measurable nodal disease and were not eligible for treatment with purine analog-based therapy. In this study, 391 patients who had received at least one prior therapy, were enrolled and randomized to receive 420 mg of IMBRUVICA® orally once daily or ARZERRA® given intravenously. Treatment was given over a period of 24 weeks until disease progression or unacceptable toxicity. Patients randomized to the ARZERRA® group on disease progression were allowed to receive treatment with IMBRUVICA®. The primary endpoint of this study was progression-free survival and the secondary endpoint was overall survival. Following recommendations from the Independent Data Monitoring Committee (IDMC), the study was stopped earlier, as the primary endpoint as well as an important secondary endpoint of the study were met. At the planned interim analysis, patients in the IMBRUVICA® group showed a statistically significant improvement in progression-free survival, the primary endpoint of the study as well as a statistically significant improvement in overall survival, the secondary endpoint of the trial. This data confirmed the efficacy of IMBRUVICA® and gives patients with CLL, an important new treatment option. Byrd JC, Furman RR, Coutre SE, et al. N Engl J Med 2013; 369:32-42
IMBRUVICA® (Ibrutinib)
The FDA on February 12, 2014 granted accelerated approval to IMBRUVICA® for the treatment of patients with Chronic Lymphocytic Leukemia (CLL) who have received at least one prior therapy. The FDA initially granted accelerated approval in November, 2013, for the treatment of patients with Mantle Cell Lymphoma (MCL) who have received at least one prior therapy. IMBRUVICA® is an oral capsule and is a product of Pharmacyclics, Inc.
A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study Evaluating the Efficacy and Safety of Idelalisib and Rituximab for Previously Treated Patients with Chronic Lymphocytic Leukemia (CLL)
SUMMARY: PI3K delta signaling is hyperactive in B-cell malignancies and is important for the activation, proliferation, homing of malignant B cells in the lymphoid tissues and their survival. The delta isoform of PI3K enzyme is predominantly expressed in leukocytes. Idelalisib is a highly selective oral inhibitor of the enzyme phosphoinositide 3-kinase (PI3K) and specifically blocks the delta isoform of PI3K enzyme and its signaling pathway. Following promising data from Phase I trials, a Phase III study was conducted in which 220 previously treated patients with recurrent CLL, measurable lymphadenopathy and ineligible to receive chemotherapy due to comorbidities, were enrolled. Patients received first dose of RITUXAN® (Rituximab) at 375 mg/m2 and then 500 mg/m2 q2 weeks x 4, followed by RITUXAN® q4 wks x 3 for a total of 8 doses along with Idelalisib 150 mg PO BID continuously until disease progression (N=110) or along with placebo. The median age was 71 years and patients had received a median of three prior therapies. Poor prognosis patients included 44% with 17p deletion/p53 mutation and 84% who had unmutated immunoglobulin variable region heavy chain (IgVH) gene. Primary endpoint was progression-free survival (PFS). Following a recommendation by an Independent Data Monitoring Committee after an interim analysis that showed superiority of RITUXAN®/Idelalisib combination, this trial was stopped early. The PFS at 24 weeks was 93% for the RITUXAN® plus Idelalisib group compared to 46% for those treated with RITUXAN® and placebo. The median PFS for the RITUXAN®/Idelalisib combination group has not yet been reached, whereas the the median PFS for the RITUXAN®/placebo arm was 5.5 months (Hazard Ratio [HR] = 0.15; P < .0001). Further, the PFS was favorable in the poor prognosis patients with either a 17p deletion or p53 mutation, when Idelalisib was combined with RITUXAN® (HR = 0.12). An improvement in the Overall Survival (OS) was also noted in the Idelalisib group compared with patients in the RITUXAN® alone group (HR = 0.28; P = 0.018). The combination of Idelalisib and RITUXAN® had an overall response rate of 81% compared with 13% in the RITUXAN®alone group (P <0 .0001). Patients treated with a combination of Idelalisib and RITUXAN® also had a higher decrease in lymphadenopathy (93%) compared with 4% in the RITUXAN® alone group (P < 0.0001). The most common adverse events which included pyrexia, fatigue, nausea and chills were similar in both treatment groups. The authors concluded that Idelalisib plus RITUXAN® may be a new treatment option for patients with previously treated CLL, who are not eligible for chemotherapy, as well as those with unfavorable cytogenetics. Furman RR, Sharman JP, Coutre SE, et al. Blood 2013;122:LBA-6
GAZYVA® plus LEUKERAN® – A new option for elderly patients with CLL
Obinutuzumab or GAZYVA® (GA101) is glycoengineered, fully humanized, third generation, type II anti-CD20 antibody (IgG1 monoclonal antibody) that selectivity binds to the extracellular domain of the CD20 antigen on malignant human B cells. It has enhanced antibody-dependent cellular cytotoxicity (ADCC) and strong apoptosis-inducing activity. In contrast, RITUXAN® (Rituximab) is a first generation chimeric anti-CD20 targeted monoclonal antibody. In a phase III trial, a combination of GAZYVA® plus LEUKERAN® (Chlorambucil), significantly improved Progression Free Survival in elderly patients with CLL compared to RITUXAN® plus LEUKERAN®. This study gives new life to LEUKERAN® when given in combination with CD20 targeted monoclonal antibodies and may be of value when treating elderly patients with comorbid conditions. This data was presented at the 2013 ASCO meeting.
