SUMMARY: The American Cancer Society estimates that approximately 15,720 new cases of chronic lymphocytic leukemia (CLL) will be diagnosed in 2014 and approximately 4600 patients will die from the disease. CLL is a disease of the elderly and the average age at the time of diagnosis is 72 years. There are two main types of lymphocytes, B and T lymphocytes/cells, and B-cell CLL is the most common type of leukemia in adults. Normal B-cell activation and proliferation is dependent on B-cell receptor (BCR) signaling.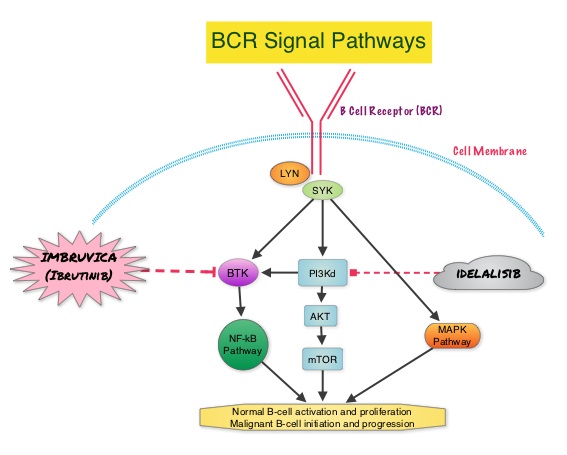 This signaling is also important for initiation and progression of B-cell lymphoproliferative disorders. Bruton’s tyrosine kinase (BTK) is a member of the Tec family of kinases, downstream of the B-cell receptor and is predominantly expressed in B-cells. It is a mediator of B-cell receptor signaling in normal and transformed B-cells. Following binding of antigen to the BCR, Syk (Spleen Tyrosine Kinase), Lyn (member of the Src family of protein tyrosine kinases) and BTK (Bruton’s Tyrosine Kinase) are activated, with subsequent propagation through PI3K/Akt, MAPK, and NF-κB pathways and resulting B-cell activation and proliferation. IMBRUVICA® (Ibrutinib) is an oral, irreversible inhibitor of BTK and inhibits cell proliferation and promotes programmed cell death (Apoptosis). The RESONATE trial is a multicenter, randomized, open-label Phase III study in which single agent IMBRUVICA® was compared to single agent ARZERRA® (Ofatumumab) in patients with relapsed or refractory CLL or Small Lymphocytic Lymphoma (SLL). In this study, 391 patients who had measurable nodal disease and received at least one prior therapy, were randomized to receive 420 mg of IMBRUVICA® orally once daily until progression (N=195) or ARZERRA® at an initial dose of 300 mg followed by 11 doses at 2000 mg, given intravenously weekly (N=196). Patients randomized to the ARZERRA® group, on disease progression were allowed to receive treatment with IMBRUVICA®. The median age was 67 years, 40% of the patients enrolled in the study were 70 years of age or over and 30% of patients had deletion of chromosome 17p. The primary endpoint of this study was Progression-Free Survival (PFS) and the secondary endpoints included Overall Survival (OS), Overall Response rate (ORR) and safety. Following recommendations from the Independent Data Monitoring Committee (IDMC), the study was stopped earlier, as the primary endpoint as well as an important secondary endpoint of the study, were met. At a median follow up of 9.4 months, IMBRUVICA® significantly prolonged PFS compared to ARZERRA® (median not reached vs 8.1 months; HR 0.215, P<0.0001) with a 78.5% reduction in the risk of disease progression and also significantly improved OS (median not reached, HR 0.43, P=0.0049) when compared with ARZERRA®, with a 57% reduction in the risk of death. The Overall Response Rates were significantly higher in the IMBRUVICA® group compared to the ARZERRA® group (42.6% vs 4.1% (P <0 .0001). An additional 20% of patients treated with IMBRUVICA® had a partial response of their persistent lymphocytosis. The benefit with IMBRUVICA® was similarly high even in the two very high risk groups of patients such as those with 17p deletions and those refractory to purine analog chemoimmunotherapy. The overall survival was significant despite the crossover of 57 patients upon progression, from the ARZERRA® group to IMBRUVICA®. Treatment was well tolerated in both groups. Diarrhea, fatigue, nausea and atrial fibrillation were more frequent in the IMBRUVICA® group but did not result in frequent dose reductions or treatment discontinuation. The authors concluded that IMBRUVICA® significantly improved Progression Free Survival, Overall Survival and Overall Response Rates, in patients with relapsed/refractory CLL/SLL, compared with ARZERRA® and IMBRUVICA® should also be a consideration for elderly patients who often are unable to tolerate intensive chemotherapy. Byrd JC, Brown JR, O’Brien SM, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr LBA7008)
This signaling is also important for initiation and progression of B-cell lymphoproliferative disorders. Bruton’s tyrosine kinase (BTK) is a member of the Tec family of kinases, downstream of the B-cell receptor and is predominantly expressed in B-cells. It is a mediator of B-cell receptor signaling in normal and transformed B-cells. Following binding of antigen to the BCR, Syk (Spleen Tyrosine Kinase), Lyn (member of the Src family of protein tyrosine kinases) and BTK (Bruton’s Tyrosine Kinase) are activated, with subsequent propagation through PI3K/Akt, MAPK, and NF-κB pathways and resulting B-cell activation and proliferation. IMBRUVICA® (Ibrutinib) is an oral, irreversible inhibitor of BTK and inhibits cell proliferation and promotes programmed cell death (Apoptosis). The RESONATE trial is a multicenter, randomized, open-label Phase III study in which single agent IMBRUVICA® was compared to single agent ARZERRA® (Ofatumumab) in patients with relapsed or refractory CLL or Small Lymphocytic Lymphoma (SLL). In this study, 391 patients who had measurable nodal disease and received at least one prior therapy, were randomized to receive 420 mg of IMBRUVICA® orally once daily until progression (N=195) or ARZERRA® at an initial dose of 300 mg followed by 11 doses at 2000 mg, given intravenously weekly (N=196). Patients randomized to the ARZERRA® group, on disease progression were allowed to receive treatment with IMBRUVICA®. The median age was 67 years, 40% of the patients enrolled in the study were 70 years of age or over and 30% of patients had deletion of chromosome 17p. The primary endpoint of this study was Progression-Free Survival (PFS) and the secondary endpoints included Overall Survival (OS), Overall Response rate (ORR) and safety. Following recommendations from the Independent Data Monitoring Committee (IDMC), the study was stopped earlier, as the primary endpoint as well as an important secondary endpoint of the study, were met. At a median follow up of 9.4 months, IMBRUVICA® significantly prolonged PFS compared to ARZERRA® (median not reached vs 8.1 months; HR 0.215, P<0.0001) with a 78.5% reduction in the risk of disease progression and also significantly improved OS (median not reached, HR 0.43, P=0.0049) when compared with ARZERRA®, with a 57% reduction in the risk of death. The Overall Response Rates were significantly higher in the IMBRUVICA® group compared to the ARZERRA® group (42.6% vs 4.1% (P <0 .0001). An additional 20% of patients treated with IMBRUVICA® had a partial response of their persistent lymphocytosis. The benefit with IMBRUVICA® was similarly high even in the two very high risk groups of patients such as those with 17p deletions and those refractory to purine analog chemoimmunotherapy. The overall survival was significant despite the crossover of 57 patients upon progression, from the ARZERRA® group to IMBRUVICA®. Treatment was well tolerated in both groups. Diarrhea, fatigue, nausea and atrial fibrillation were more frequent in the IMBRUVICA® group but did not result in frequent dose reductions or treatment discontinuation. The authors concluded that IMBRUVICA® significantly improved Progression Free Survival, Overall Survival and Overall Response Rates, in patients with relapsed/refractory CLL/SLL, compared with ARZERRA® and IMBRUVICA® should also be a consideration for elderly patients who often are unable to tolerate intensive chemotherapy. Byrd JC, Brown JR, O’Brien SM, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr LBA7008)

