SUMMARY: The American Cancer Society estimates that approximately 18,960 new cases of Chronic Lymphocytic Leukemia (CLL) were diagnosed in 2016 and approximately 4660 patients died from the disease. CLL is a disease of the elderly and the average age at the time of diagnosis is 72 years. A new treatment algorithm for the upfront treatment of CLL divides treatment naïve CLL patients into three groups and therapy should be chosen accordingly:
GROUP 1 consists of elderly patients with comorbidities who are unfit for aggressive interventions. The goal of therapy for this patient group should be to minimize toxicity rather than achieve long term remissions. In elderly CLL patients with comorbid conditions, Chlorambucil was often considered as a standard first-line therapy because of the higher rate of toxicities associated with FLUDARA® (Fludarabine) and TREANDA® (Bendamustine). The preferred choice for this group now is IMBRUVICA® (Ibrutinib). IMBRUVICA® is an oral, irreversible inhibitor of BTK and inhibits cell proliferation and promotes programmed cell death (Apoptosis) by blocking B-cell activation and signaling.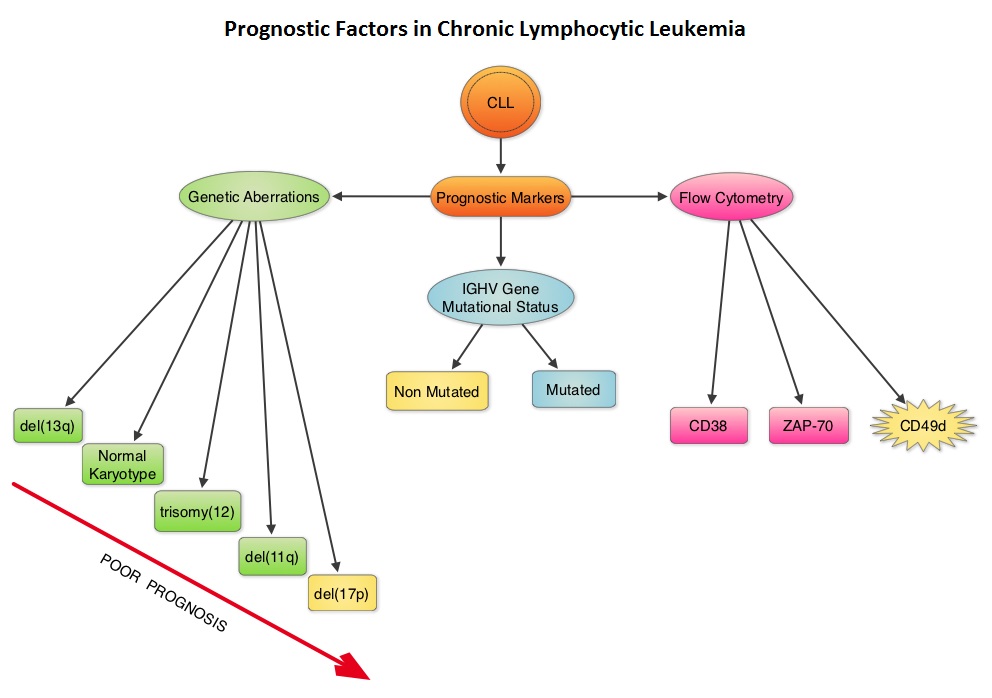
In CLL patients 65 years or older with relapsed or refractory disease (94%), or who were treatment-naive (85%), long term treatment and follow up with single-agent IMBRUVICA® resulted in high response rates across all subgroups of patients. It was noted that there has been increasing Complete Response Rate with each follow up in the treatment naïve group with a Complete Response Rate of 26%. (2015 AACR Annual Meeting. Abstract CT132). Of the 94 patients treated with IMBRUVICA®, at 45 months of follow up, the Progression Free Survival (PFS) at 30 months was 96% and Overall Survival (OS) rate was 96% in treatment-naive group, whereas the 30 month PFS was 76% and OS rate was 87% in relapsed/refractory patient group. The median PFS in patients with del(17p) was 32.4 month compared to 12 months with previously published best front line therapies.
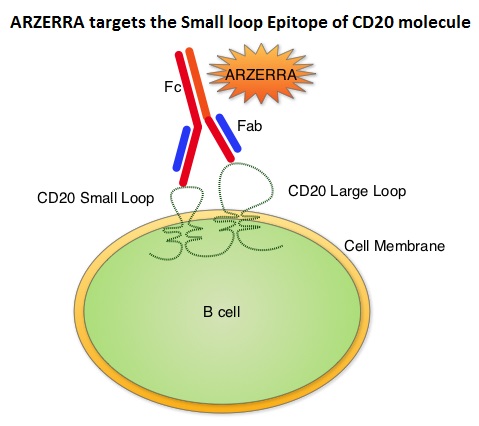
In the RESONATE trial, which compared IMBRUVICA® to ARZERRA® (Ofatumumab) in patients with relapsed/refractory CLL (N Engl J Med 371:213-223, 2014), median PFS was not reached with IMBRUVICA® compared to a median PFS of 8.1 months with ARZERRA®, representing a 78% reduction in the risk of progression (HR=0.22; P<0.001). IMBRUVICA® also significantly improved Overall Survival (HR=0.43; P=0.005). The Overall Response Rate was also significantly higher in the IMBRUVICA® group than in the ARZERRA® group (42.6% versus 4.1%; P<0.001). Toxicities with IMBUVICA® are mild and include diarrhea and ecchymoses. Atrial fibrillation is seen in approximately 8% of elderly patients and needs attention.
GROUP 2 includes young fit patients with IGVH mutations for whom more aggressive combination therapy should be recommended for long term benefit. This group of patients should be offered three drug regimen consisting of FCR – FLUDARA®/Cyclophosphamide/RITUXAN® (Rituximab). The long term follow up of the pivotal FCR300 study revealed that 40% of patients were progression free at 14 years with a plateau noted in the PFS curve (Blood 127:303-309, 2016). About 60% of the patients with the IGVH mutation who were negative for Minimal Residual Disease (MRD) at the end of treatment, were progression free at 14 years. It was also demonstrated in a subsequent cohort that testing negative for Minimal Residual Disease after a response to FCR regimen was the most important determinant of Progression Free and Overall Survival. Among responders to FCR who were MRD negative, there was only one progression and no deaths after 4 years. Patients with IGVH-mutated disease had a 2.7-fold higher chance of being MRD negative after treatment with FCR. Bendamustine/RITUXAN® regimen is also often recommended for young and fit patients with Chronic Lymphocytic Leukemia, as the risk of neutropenia and infections are lower. However, FCR is associated with longer remissions by at least one year compared to Bendamustine/RITUXAN®.
GROUP 3 comprises of fit patients who are IGVH mutation-negative and will not achieve long remissions with combination chemotherapy. FCR regimen will not achieve 10-15 year remissions in this patient group and these patients should either be treated with IMBRUVICA® or enrolled in clinical trials.
ZYDELIG® (Idelalisib) is a highly selective oral inhibitor of the enzyme phosphoinositide 3-kinase (PI3K) and specifically blocks the delta isoform of PI3K enzyme and its signaling pathway. In the pivotal trial of previously treated patients with recurrent CLL (N Engl J Med 370:997-1007, 2014), the median PFS for the RITUXAN®/ ZYDELIG® combination group has not yet been reached, whereas the median PFS for the RITUXAN®/placebo arm was 5.5 months (HR=0.15; P<0.0001). This suggested an 85% reduction in the risk of progression. Further, the PFS was favorable in the poor prognosis patients with either a 17p deletion or p53 mutation, when ZYDELIG® was combined with RITUXAN® (HR=0.12). An improvement in the Overall Survival (OS) was also noted in the ZYDELIG® group compared with patients in the RITUXAN® alone group (HR=0.28; P=0.018). The combination of ZYDELIG® and RITUXAN® had an Overall Response Rate of 81% compared with 13% in the RITUXAN® alone group (P<0 .0001). Patients treated with a combination of ZYDELIG® and RITUXAN® also had a higher decrease in lymphadenopathy (93%) compared with 4% in the RITUXAN® alone group (P<0.0001). Lymphocytosis resolves more slowly with ZYDELIG® than with IMBRUVICA® and therefore, ZYDELIG® is combined with RITUXAN®. ZYDELIG® should not be used as first line therapy due to associated toxicities such as colitis, elevated transaminases and pneumonitis seen more so in treatment naïve patients, as these patients have less T-cell depletion.
Chronic Lymphocytic Leukemia with del(17p)
IMBRUVICA® is the preferred first line therapy. VENCLEXTA® (Venetoclax) is a second generation, oral, selective, small molecule inhibitor of BCL2 and restores the apoptotic processes in tumor cells. In a pivotal study, VENCLEXTA® monotherapy in patients with Relapsed/Refractory del(17p) CLL resulted in a ORR of 79.4% with 8% Complete Responses and some patients attained MRD-negative status in their peripheral blood which is remarkable (N Engl J Med 374:311-322, 2016). The median Duration of Response (DoR) has not been reached. This drug should be considered as second line therapy.
Dr O’Brien concluded that this new evidence based treatment algorithm stratifies patients differently from the traditional algorithm. O’Brien SM: How do we sequence the best treatment options for patients with CLL based on age and prognostic features? 2016 Pan Pacific Lymphoma Conference. Presented July 22, 2016. Koloa, HI, United States

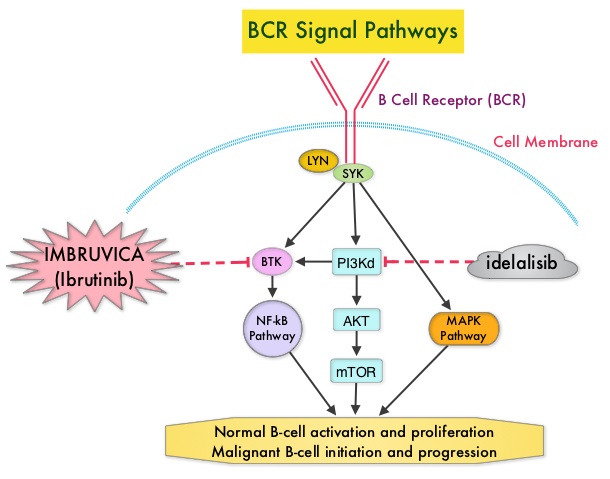
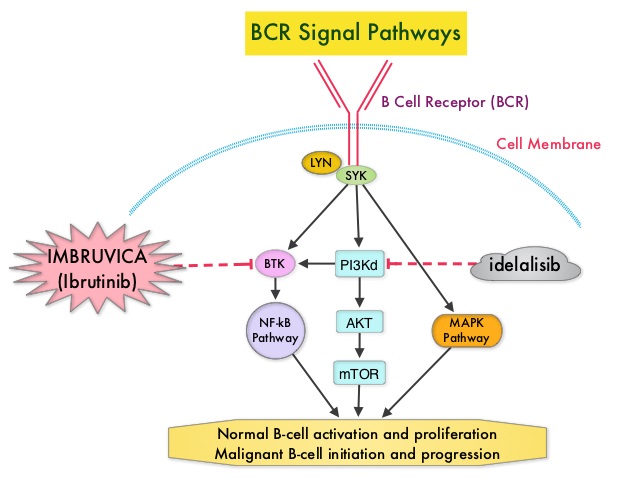
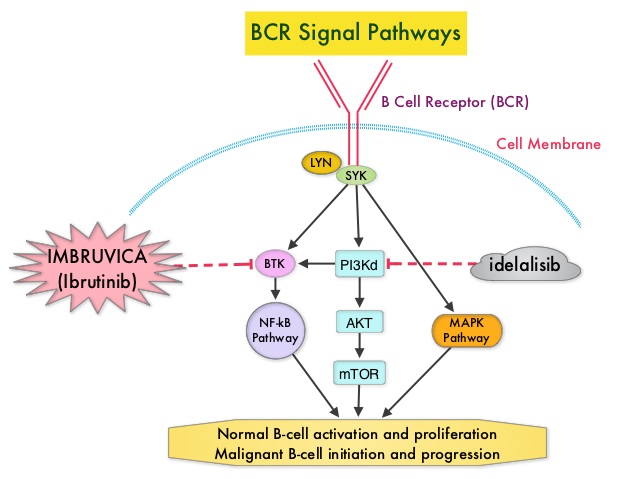
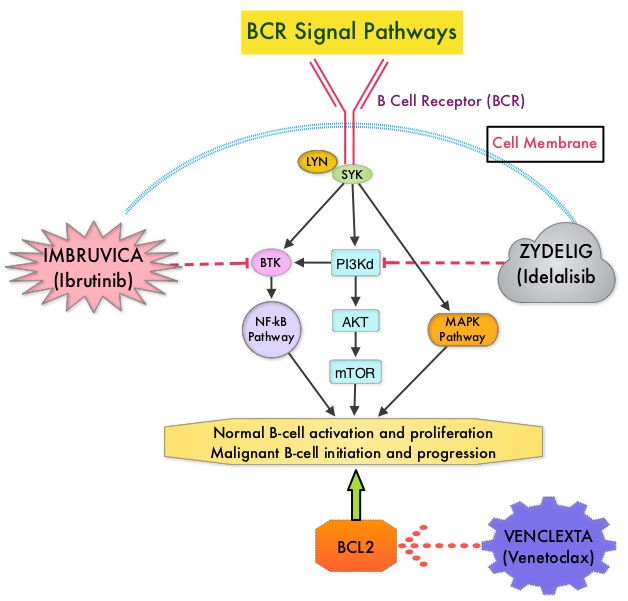 Following binding of antigen to the B-Cell Receptor, kinases such as Syk (Spleen Tyrosine Kinase), Lyn (member of the Src family of protein tyrosine kinases) and BTK (Bruton's Tyrosine Kinase) are activated, with subsequent propagation through PI3K/Akt, MAPK, and NF-κB pathways. This results in B-cell activation and proliferation. PI3K (PhosphoInositide 3-Kinase) delta signaling, is hyperactive in B-cell malignancies and is important for the activation, proliferation, homing of malignant B cells in the lymphoid tissues and their survival. The delta isoform of PI3K enzyme is predominantly expressed in leukocytes. Targeting proteins in key pathways of B-cell biology has fundamentally changed the management and outcomes of CLL, over the past 5 years. IMBRUVICA® (Ibrutinib) is an oral, irreversible inhibitor of BTK and inhibits cell proliferation and promotes programmed cell death (Apoptosis) by blocking B-cell activation and signaling. ZYDELIG® (Idelalisib) is a highly selective oral inhibitor of the enzyme PI3K and specifically blocks the delta isoform of PI3K enzyme and its signaling pathway. The pro-survival (anti-apoptotic) protein BCL2, is over expressed by CLL cells and regulates clonal selection and cell survival. A new class of anticancer agents known as BH3-mimetic drugs are in development that mimic the activity of the physiologic antagonists of BCL2 and related proteins and promote apoptosis (programmed cell death).
Following binding of antigen to the B-Cell Receptor, kinases such as Syk (Spleen Tyrosine Kinase), Lyn (member of the Src family of protein tyrosine kinases) and BTK (Bruton's Tyrosine Kinase) are activated, with subsequent propagation through PI3K/Akt, MAPK, and NF-κB pathways. This results in B-cell activation and proliferation. PI3K (PhosphoInositide 3-Kinase) delta signaling, is hyperactive in B-cell malignancies and is important for the activation, proliferation, homing of malignant B cells in the lymphoid tissues and their survival. The delta isoform of PI3K enzyme is predominantly expressed in leukocytes. Targeting proteins in key pathways of B-cell biology has fundamentally changed the management and outcomes of CLL, over the past 5 years. IMBRUVICA® (Ibrutinib) is an oral, irreversible inhibitor of BTK and inhibits cell proliferation and promotes programmed cell death (Apoptosis) by blocking B-cell activation and signaling. ZYDELIG® (Idelalisib) is a highly selective oral inhibitor of the enzyme PI3K and specifically blocks the delta isoform of PI3K enzyme and its signaling pathway. The pro-survival (anti-apoptotic) protein BCL2, is over expressed by CLL cells and regulates clonal selection and cell survival. A new class of anticancer agents known as BH3-mimetic drugs are in development that mimic the activity of the physiologic antagonists of BCL2 and related proteins and promote apoptosis (programmed cell death).