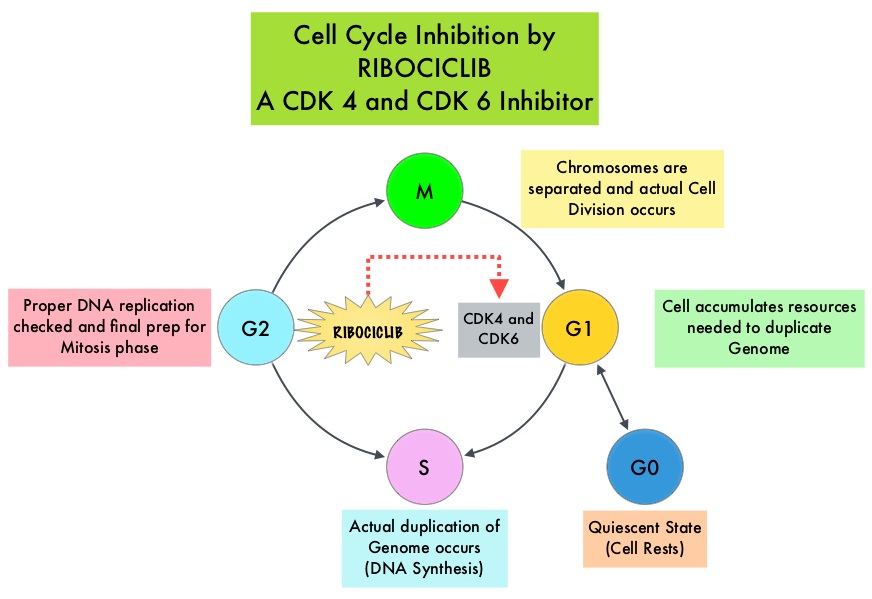
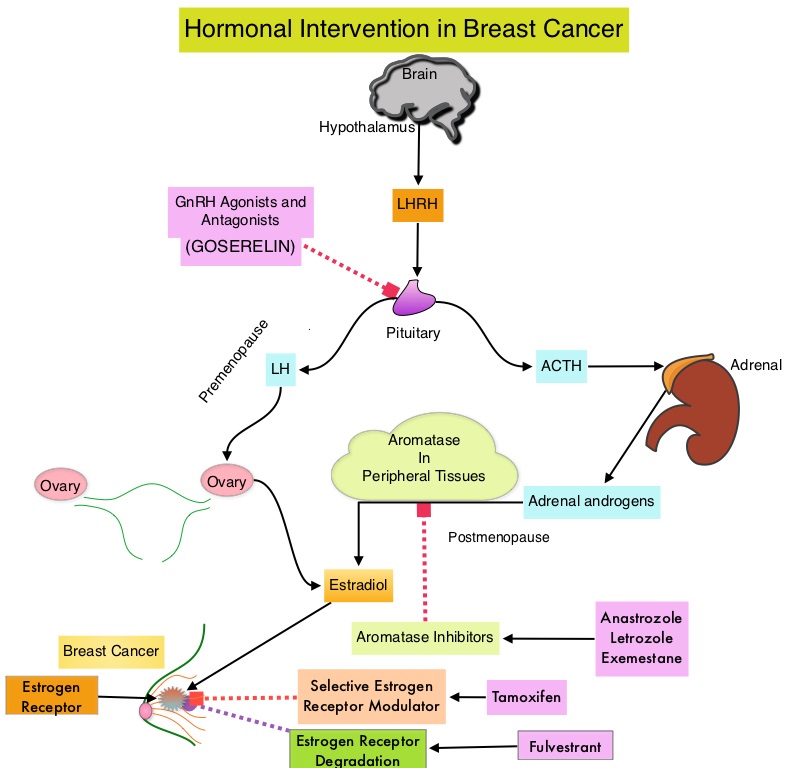
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately, 255,180 new cases of invasive breast cancer will be diagnosed in 2017 and over 41,070 women will die of the disease. The National Surgical Adjuvant Breast and Bowel Project (NASBP) protocols B-04 and B-06 have clearly established after more than a 2 decades of evaluation and follow up that, in Stage I and II breast cancer, there is no significant difference in either distant Disease Free or Overall Survival between the Breast Conserving Therapy (BCT) and Breast Removal Surgery (Mastectomy). This data established Breast Conserving Therapy (BCT) as the preferred local-regional procedure. These trials however often excluded elderly patients or patients with co-morbidities. Radiotherapy after breast- conserving surgery significantly decreases the risk of local recurrence and improves Breast Cancer Specific Survival (BCSS) in certain subgroups of patients. According to the American Cancer Society, 42% of all invasive breast cancers in the US occur in women 65 years of age or older. These patients may have associated co-morbidities and may therefore be appropriate candidates for breast- conserving surgery rather than mastectomy. These patients also have better outcomes, as post-op recovery time is shorter. There is however limited data to confirm these findings, as most studies evaluated limited numbers of patients, lacked long term follow up and the cause of death in these patients could not be clearly determined.
To further address this question, the authors in this study compared breast-conserving surgery plus radiation therapy (BCT) with Mastectomy, for Breast Cancer Specific (BCSS) and Overall Survival (OS), in a population-based study of 129,692 breast cancer patients without metastatic disease, in the Netherlands. Patients were selected from the Netherlands Cancer Registry, who had T1-2, N0-2, M0 breast cancer, diagnosed between1999 and 2012. Patients were divided into two time cohorts: those diagnosed between 1999 and 2005 (long term follow up), and those diagnosed between 2006 and 2012, (contemporary adjuvant systemic therapy). The influence of prognostic factors such as age, stage, adjuvant systemic therapy, hormonal and HER2 receptor status and co-morbidities was studied in these two groups of patients, in order to identify possible prognostic factors, that might predict patient groups, who could benefit the most from Breast Conserving Therapy (BCT). Information on the cause of death was obtained from Statistics Netherlands, also known as the Dutch Central Bureau of Statistics.
It was noted that for patients in the long-term follow up cohort, Breast Conservation Therapy was associated with a statistically significant improvement in Breast Cancer Specific Survival and Overall Survival compared to Mastectomy in all T1-2, N0-2 stages. For patients diagnosed between 2006 to 2012 (contemporary adjuvant systemic therapy), Breast Conserving Therapy was again associated with a statistically significant improvement in Breast Cancer Specific Survival and Overall Survival for patients in the T1-2, N0-1 stage but not those with T1-2, N2 disease, and in this later group, Breast Cancer Specific Survival (BCSS) with conservation therapy was equal to that with mastectomy. Subgroup analyses in the T1-2, N0-1 subset showed superior BCSS with breast conservation in patients older than 50 years, those who did not receive chemotherapy and those who had co-morbid conditions, irrespective of hormone receptor or HER2 status. The Overall Survival (OS) results were similar. Among patients younger than 50 years of age without co-morbidities, and those who received chemotherapy, BCSS with breast conservation was equal to that with mastectomy, but OS was better with Breast Conservation Therapy than with Mastectomy.
It was concluded that in this large population of “real world” patients as seen in daily clinical practice, Breast Conserving Therapy is associated with superior Breast Cancer-Specific and Overall Survival when compared to Mastectomy in patients over 50 years of age, T1-2, N0-1 M0 stage, patients who had not received chemotherapy and patients with co-morbidities. This benefit was confirmed for patients in both time cohorts. This study information allows the Health Care Provider to decide which type of surgical treatment is best suited for some subtypes of Breast cancer. Breast conserving therapy and mastectomy revisited: Breast cancer-specific survival and the influence of prognostic factors in 129,692 patients. Lagendijk M, van Maaren MC, Saadatmand S, et al. ECCO2017 European Cancer Congress. Abstract number: 4LBA