The FDA on September 28, 2017 approved VERZENIO® in combination with Fulvestrant for women with HR-positive, HER2-negative advanced or metastatic breast cancer, with disease progression following endocrine therapy. In addition, VERZENIO® was approved as monotherapy, for women and men with HR-positive, HER2-negative advanced or metastatic breast cancer, with disease progression following endocrine therapy and prior chemotherapy in the metastatic setting. VERZENIO® is a product of Eli Lilly and Company.
Tag: Breast Cancer
FDA Approves NERLYNX® for Adjuvant Treatment of HER2 Positive Breast Cancer
The FDA on July 17, 2017 approved NERLYNX® (Neratinib) for the extended adjuvant treatment of adult patients with early stage HER2-overexpressed/amplified breast cancer, to follow adjuvant Trastuzumab (HERCEPTIN®)-based therapy. NERLYNX® is a potent, irreversible, oral Tyrosine Kinase Inhibitor, of HER1, HER2 and HER4 (pan-HER inhibitor). NERLYNX® is the first TKI approved by the FDA, shown to reduce the risk for disease recurrence, in patients with early stage HER2-positive breast cancer and demonstrated significantly improved 2-year invasive Disease Free Survival.
Screening Mammography Starting at Age 40 years May Reduce Breast Cancer Deaths by 40 percent
In the US, about 33 million screening mammograms are performed each year. Currently, the major national health care organizations in the US have different recommendations for screening mammography which has led to some confusion and emotional counterarguments. These several different recommendations include 1) Annual screening at ages 40 to 84 years. 2) Annual screening at ages 45 to 54 years and then biennially at ages 55 to 79 years. 3) Biennial screening at ages 50 to 74 years.
In a recently published study (CANCER, August 21, 2017), it was noted that the greatest breast cancer-specific mortality reduction was achieved with annual screening of women starting at age 40 years, saving 29,369 lives from breast cancer. This is the first study to compare the three most widely discussed recommendations for screening mammography, head to head.
Screening Mammography Starting at Age 40 years May Reduce Breast Cancer Deaths by 40 percent
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. It is estimated that 252,710 new cases of invasive breast cancer and 63,410 new cases of non-invasive breast cancer will be diagnosed in women in 2017 and 40,610 women are expected to die from the disease. In the US, about 33 million screening mammograms are performed each year.
Currently, the major national health care organizations in the US have different recommendations for screening mammography which has led to some confusion and emotional counterarguments. These several different recommendations include 1) Annual screening at ages 40 to 84 years 2) Annual screening at ages 45 to 54 years and then biennially at ages 55 to 79 years 3) Biennial screening at ages 50 to 74 years.
To address this varied recommendations and help women make informed decisions regarding mammography screening, the authors used computer modeling (CISNET models) to assess the three major screening mammography recommendations, and estimate the number of breast cancer deaths that might be prevented with the different screening mammography schedules. Cancer Intervention and Surveillance Modeling Network (CISNET) is a consortium of NCI-sponsored investigators who use statistical modeling to improve understanding of cancer control interventions in prevention, screening and treatment, and their effects on population trends in incidence and mortality. CISNET has been cited by the International Society Pharmacoeconomics and Outcomes Research (ISPOR) Task Force on Good Modeling Practices for its role in establishing a forum that enables researchers to compare results and articulate reasons for discrepancies.
It was noted in this study that the mean mortality reduction in breast cancer-specific deaths was greatest with the recommendation of annual screening at ages 40 to 84 years (39.6%), which meant that 29,369 lives were saved from breast cancer, compared with the recommendation of screening annually at ages 45 to 54 years, then biennially at ages 55 to 79 years (30.8%), which meant that 22,829 were lives saved from breast cancer, and the recommendation of biennial screening at ages 50 to 74 years (23.2%) which meant that 17,153 lives were saved from breast cancer.
The study also took into consideration risks associated with screening, including callbacks for additional imaging following indeterminate or suspicious mammographic finding and in some cases, a breast biopsy, only to find out that the findings were benign. The authors commented that the average woman in her 40s getting annual screening can expect additional and unnecessary screening about once every 12 years and unnecessary breast biopsy recommendations once every 150 years. Other rare risks with screening mammography include breast cancer that could be missed and breast cancer caused by mammogram radiation.
It was concluded that based on the CISNET models, the greatest breast cancer-specific mortality reduction is achieved with annual screening of women starting at age 40 years. They added that this is the first study to compare the three most widely discussed recommendations for screening mammography, head to head. These findings will guide women and their Health Care Providers in deciding when to begin screening mammography and how often to get screened. Comparison of recommendations for screening mammography using CISNET models. Arleo EK, Hendrick E, Helvie MA, et al. CANCER; Published Online: August 21, 2017. http://doi.wiley.com/10.1002/cncr.30842
FDA Approves NERLYNX® for Adjuvant Treatment of HER2 Positive Breast Cancer
SUMMARY: The FDA on July 17, 2017 approved NERLYNX® (Neratinib) for the extended adjuvant treatment of adult patients with early stage HER2-overexpressed/amplified breast cancer, to follow adjuvant Trastuzumab (HERCEPTIN®)-based therapy. Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately, 255,180 new cases of invasive breast cancer will be diagnosed in 2017 and over 41,070 women will die of the disease. The HER or erbB family of receptors consist of HER1, HER2, HER3 and HER4. Approximately 15%-20% of invasive breast cancers overexpress HER2/neu oncogene, which is a negative predictor of outcomes without systemic therapy. HERCEPTIN® (Trastuzumab) is a humanized monoclonal antibody targeting HER2 and adjuvant chemotherapy given along with HERCEPTIN® reduces the risk of disease recurrence and death, among patients with HER2-positive, early breast cancer. Nonetheless, approximately 25% of patients will develop recurrent disease within 10 years following this adjuvant intervention. Extending the duration of adjuvant HERCEPTIN® therapy or adding TYKERB® (Lapatinib), a Tyrosine Kinase Inhibitor that targets HER1 and HER2, has not improved outcomes.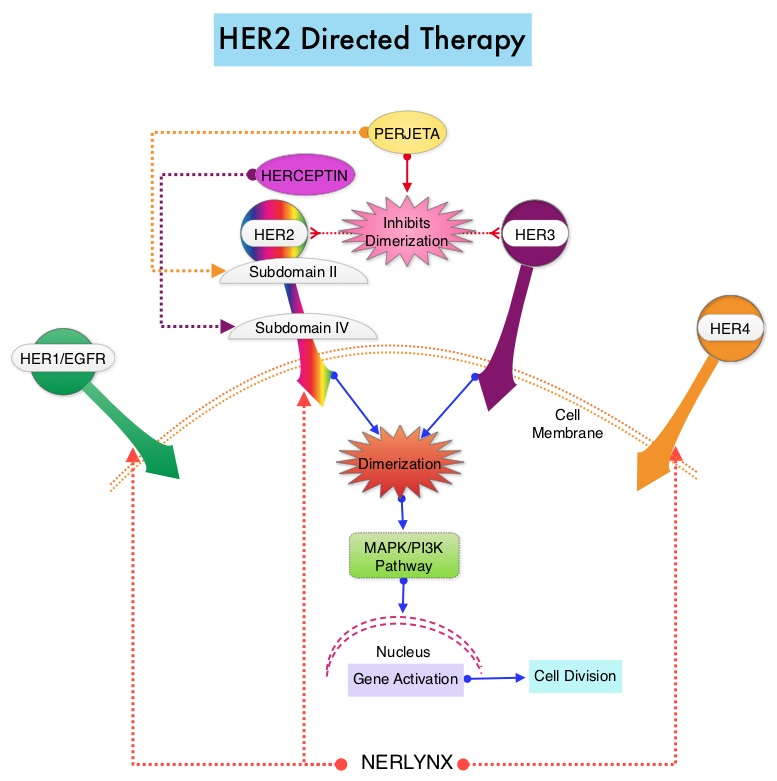
NERLYNX® is a potent, irreversible, oral Tyrosine Kinase Inhibitor, of HER1, HER2 and HER4 (pan-HER inhibitor). NERLYNX® interacts with the catalytic domain of HER1, HER2, and HER4 and blocks their downstream signaling pathways, resulting in decreased cell proliferation and increased cell death. Clinical data has suggested that NERLYNX® has significant activity in suppressing HER-mediated tumor growth and is able to overcome tumor escape mechanisms experienced with current HER2-targeted and chemotherapeutic agents. It has been well known that hormone receptor positive breast cancer patients, who are also HER2-positive, have relative resistance to hormone therapy. Preclinical models had suggested that the addition of NERLYNX® could improve responses in ER positive, HER2-positive breast cancer patients. Further, NERLYNX® has clinical activity in patients with HER2-positive metastatic breast cancer.
The approval of NERLYNX® was based on ExteNET trial, which is a multicentre, randomized, double-blind, placebo-controlled, phase III study, in which the efficacy and safety of 12 months of NERLYNX® after HERCEPTIN®-based adjuvant therapy was evaluated, in patients with early stage HER2-positive breast cancer. Patients with early stage HER2-positive breast cancer (N=2,840), and within two years of completing adjuvant HERCEPTIN®, were randomized in a 1:1 ratio to receive either oral NERLYNX® 240 mg per day (N=1420) or placebo (N=1420), for one year. Patients were stratified by hormone receptor status, nodal status (0, 1-3, or 4 or more), and HERCEPTIN® adjuvant regimen (sequentially versus concurrently with chemotherapy). The Primary endpoint was invasive Disease Free Survival (iDFS), defined as the time between the randomization date to the first occurrence of invasive recurrence (local/regional, ipsilateral or contralateral breast cancer), distant recurrence, or death from any cause, within two years of follow up. The median follow up was 2 years.
In the updated analysis, the two year iDFS was 94.2% in patients treated with NERLYNX® compared with 91.9% in those receiving placebo (HR 0.66; P=0.008). Patients with ER positive breast cancer were noted to have greater benefit. The most common grade 3-4 adverse events associated with NERLYNX® were diarrhea, vomiting and nausea. Patients can experience diarrhea early, in the first 2 or 3 days and this can be alleviated using antidiarrheal prophylaxis with Loperamide, initiated with the first dose of NERLYNX® and continued for the first 2 months of treatment and as needed thereafter.
It was concluded that NERLYNX® when given for 12 months after chemotherapy and HERCEPTIN®-based adjuvant therapy, to women with HER2-positive breast cancer, significantly improved 2-year invasive Disease Free Survival. Longer follow up will hopefully address if there is an Overall Survival benefit with this treatment intervention. NERLYNX® is the first TKI approved by the FDA, shown to reduce the risk for disease recurrence, in patients with early stage HER2-positive breast cancer. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial Chan A, Delaloge S, Holmes FA, et al. The Lancet Oncology 2016; 17:367- 377
NERLYNX ® (Neratinib)
The FDA on July 17, 2017 approved NERLYNX ® for the extended adjuvant treatment of adult patients with early stage HER2-overexpressed/amplified breast cancer, to follow adjuvant Trastuzumab-based therapy. NERLYNX ® is a product of Puma Biotechnology, Inc.
Weight Gain Increases the Risk for Postmenopausal Breast cancer
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately, 255,180 new cases of invasive breast cancer will be diagnosed in 2017 and over 41,070 women will die of the disease. Obesity is an important contributing factor to postmenopausal breast cancer incidence and mortality. Based on recently published meta-analysis, in women diagnosed with breast cancer, there is an approximately 30% increased risk of disease recurrence or death in those who are obese compared to those with ideal body weight. Increasing physical activity may lower the risk of breast cancer recurrence. According to the consensus from the St Gallen Consensus Conference in 2015, obesity has been associated with poor breast cancer outcomes. Obesity is associated with alterations in insulin/glucose homeostasis, adipokines, and sex hormones, which may play a role in breast cancer outcomes. Weight loss can lead to reductions in C-reactive protein, insulin, glucose, and leptin. These mediators have all been implicated to have prognostic significance in breast cancer. 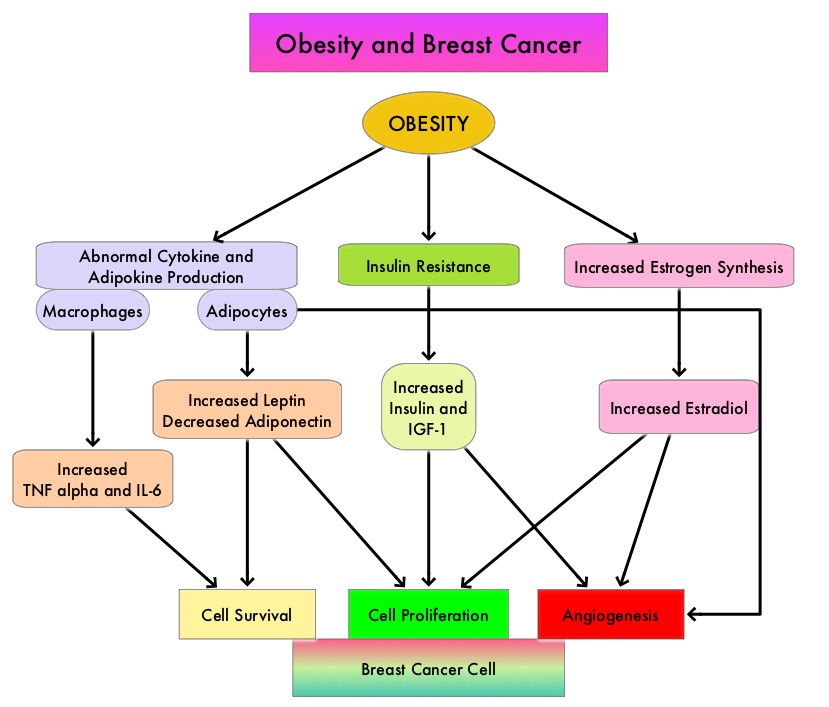
The Nurses’ Health Studies (NHS) are the largest and longest running investigations focused on women’s health. This was established in 1976 and the information provided by its 238,000 dedicated nurse-participants has allowed NHS to produce key advances impacting women’s health. These studies are conducted by researchers at Harvard School of Public Health and Brigham and Women's Hospital in Boston, Massachusetts. The authors conducted a clinical trial in this NHS cohort and studied the effects of weight and weight changes in early adulthood and risk of breast cancer later in life. A prospective observational study was conducted among 74,177 women from the Nurses' Health Study from 1980-2012. These women provided information on breast cancer risk factors such as reproductive factors, hormone therapy, anthropometric variable, benign breast disease, and family history of breast cancer. This information was updated every 2 years up to the time of data analysis. Each individual’s weight at age 18 was collected in 1980.
During the observation period, 4965 cases of invasive breast cancer were reported for the 74,177 women followed from 1980 to 2012. Weight gain over a long period of time from age 18, both during premenopause and postmenopause, were positively associated with postmenopausal breast cancer risk. However, premenopausal weight gain was not related to premenopausal breast cancer risk. Further, weight gain from age 18 yrs onwards was positively associated with ER+/PR+ postmenopausal breast cancer and there was a 50% increased risk for breast cancer with a weight gain of 30 kg. This direct association was not seen for ER+/PR- or ER-/PR- breast cancer. The authors noted that overall, 17% of ER+/PR+ postmenopausal breast cancer and 14% of total postmenopausal breast cancer are attributable to weight gain of more than 5 kg after age 18.
It was concluded that 14% of postmenopausal breast cancer could be prevented if women avoided excessive weight gain of more than 5 kg after age 18. This study adds new insights on weight gain during premenopausal years and risk for postmenopausal breast cancer. Weight and weight changes in early adulthood and later breast cancer risk. Rosner B, Eliassen AH, Toriola AT, et al. Int J Cancer. 2017 Jan 30. doi: 10.1002/ijc.30627 [Epub ahead of print]
Late Breaking Abstract – ASCO 2017 Adjuvant Dual HER2 Regimen of PERJETA® and HERCEPTIN® Improves Outcomes in Early Stage Breast Cancer
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately, 255,180 new cases of invasive breast cancer will be diagnosed in 2017 and over 41,070 women will die of the disease. The HER or erbB family of receptors consist of HER1, HER2, HER3 and HER4. Approximately 15%-20% of invasive breast cancers overexpress HER2/neu oncogene, which is a negative predictor of outcomes without systemic therapy. HERCEPTIN® (Trastuzumab) is a humanized monoclonal antibody targeting HER2. Trastuzumab binds to subdomain IV of the HER2 extracellular domain and blocks the downstream cell signaling pathways (PI3K-AKT pathway) and induces Antibody Dependent Cellular Cytotoxicity (ADCC). Adjuvant chemotherapy given along with HERCEPTIN® reduces the risk of disease recurrence and death, among patients with HER2-positive early breast cancer.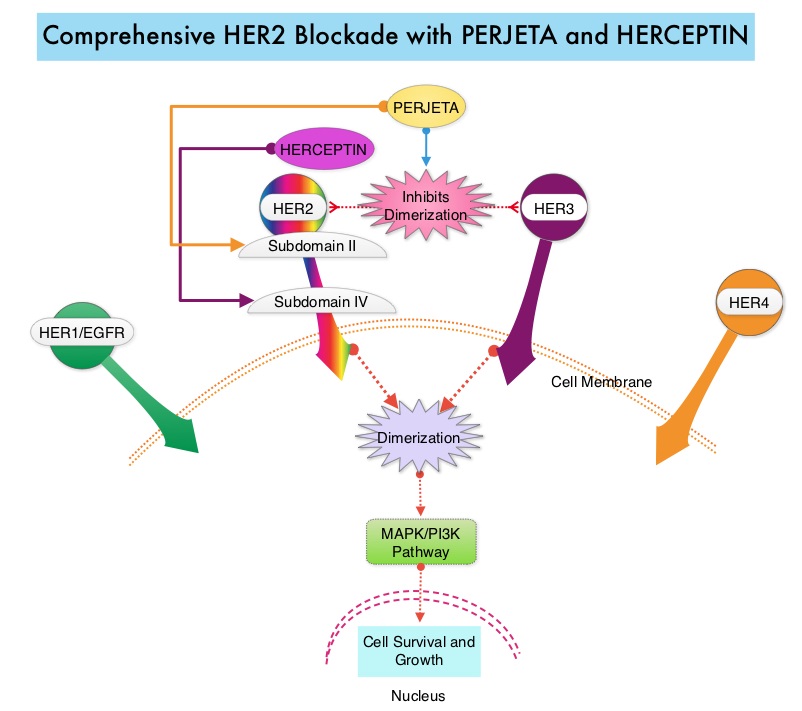
PERJETA® (Pertuzumab) is a recombinant humanized monoclonal antibody that binds to the HER2 at a different epitope of the HER2 extracellular domain (subdomain II) compared to HERCEPTIN® and prevents the dimerization of HER2 with HER3 receptor. PERJETA® induces ADCC similar to HERCEPTIN®. By combining HERCEPTIN® and PERJETA®, a more comprehensive blockade of HER2 signaling can be accomplished, as these two agents bind to different HER2 epitopes and may complement each other and improve efficacy. In the CLEOPATRA study, the addition of PERJETA® to HERCEPTIN® and Docetaxel resulted in significant improvement in Progression Free Survival (PFS) and Overall Survival (OS), in patients with HER-positive metastatic breast cancer. This triple drug combination also resulted in a significantly increased pathological Complete Response rate, when given in a neoadjuvant setting (NeoSphere trial).
Based on these previously published efficacy data, the authors in this study investigated whether the addition of PERJETA® to adjuvant HERCEPTIN® and chemotherapy, improves outcomes, among patients with HER2-positive early breast cancer. APHINITY is a prospective, randomized, multicenter, multinational, double-blind, placebo-controlled phase III trial in which a total of 4805 patients were randomly assigned in a 1:1 ratio, to receive standard adjuvant anthracycline or non-anthracycline chemotherapy regimen along with HERCEPTIN® plus either PERJETA® (2400 patients) or placebo (2405 patients). Anti-HER2 therapy was administered for a total of 1 year. Patients could receive radiotherapy and/or endocrine therapy following completion of adjuvant chemotherapy. Eligible patients had node-positive or high-risk node-negative (tumor diameter greater than 1.0 cm), HER2-positive, non-metastatic, adequately excised breast cancer. Both treatment groups were well balanced and about 37% of the patients had 1-3 positive lymph nodes and 25% of the patients had 4 or more positive lymph nodes. Two thirds of the patients were hormone receptor positive and about 78% of the patients received an anthracycline containing adjuvant chemotherapy regimen. The median follow up was 45.4 months and one year of treatment was completed by approximately 85% of the patients in both treatment groups. The primary end point was Disease Free Survival (DFS) from invasive breast cancer and secondary end points included Overall Survival (OS) and DFS from non-invasive breast cancers.
The addition of PERJETA® to chemotherapy and HERCEPTIN® resulted in a higher rate of DFS for invasive breast cancer with a 3-year invasive DFS of 94.1% in the PERJETA® group and 93.2% in the placebo group (HR=0.81; P=0.045), in favor of PERJETA®. Patients in the high risk subgroups benefited the most. The 3-year invasive DFS for patients with node-positive disease was 92.0% in the PERJETA® group, compared with 90.2% in the placebo group (HR=0.77; P=0.02). In the cohort of patients with hormone receptor negative tumors, the 3-year invasive DFS was 92.8% in the PERJETA® group and 91.2% in the placebo group (HR=0.76; P=0.08). The site of first distant recurrence was visceral or in the CNS rather than the bone. Cardiac toxicities were uncommon in both treatment groups and patients in the PERJETA® group had higher incidence of diarrhea while on concurrent chemotherapy.
It was concluded that for patients with HER2-positive early breast cancer, the addition of PERJETA® to standard postoperative HERCEPTIN® based adjuvant chemotherapy, significantly improved Disease Free Survival for invasive breast cancer. This benefit was more so for those patients with high risk disease. APHINITY trial (BIG 4-11): A randomized comparison of chemotherapy (C) plus trastuzumab (T) plus placebo (Pla) versus chemotherapy plus trastuzumab (T) plus pertuzumab (P) as adjuvant therapy in patients (pts) with HER2-positive early breast cancer (EBC). von Minckwitz G, Procter MJ, De Azambuja E, et al. J Clin Oncol. 2017;35(suppl; abstr LBA500).
Late Breaking Abstract – ASCO 2017 LYNPARZA® Improves Progression Free Survival in BRCA Positive Breast Cancer
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately, 255,180 new cases of invasive breast cancer will be diagnosed in 2017 and over 41,070 women will die of the disease. DNA can be damaged due to errors during its replication or as a result of environmental exposure to ultraviolet radiation from the sun or other toxins. The tumor suppressor genes such as BRCA1 (Breast Cancer 1) and BRCA2 help repair damaged DNA and thus play an important role in maintaining cellular genetic integrity, failing which these genetic aberrations can result in malignancies. The BRCA1 gene is located on the long (q) arm of chromosome 17 whereas BRCA2 is located on the long arm of chromosome 13. Mutations in BRCA1 and BRCA2 account for about 20 to 25 percent of hereditary breast cancers and about 5 to 10 percent of all breast cancers. These mutations can be inherited from either of the parents and a child has a 50 percent chance of inheriting this mutation and the deleterious effects of the mutations are seen even when an individual’s second copy of the gene is normal. 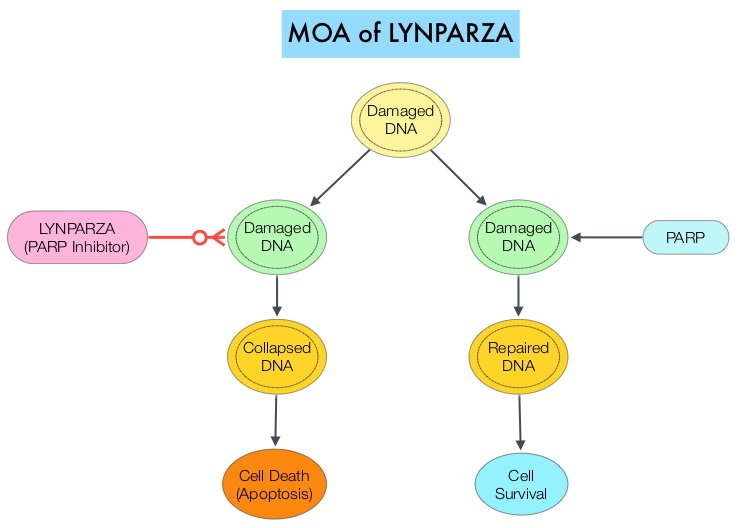
The PARP (Poly ADP Ribose Polymerase) family of enzymes which include PARP1 and PARP2, repair damaged DNA. LYNPARZA® (Olaparib) is a PARP enzyme inhibitor that causes cell death in tumors that already have a DNA repair defect, such as those with BRCA1 and BRCA2 mutations. The FDA approved LYNPARZA® (Olaparib) in 2014 as monotherapy for the treatment of patients with deleterious or suspected deleterious germline BRCA mutated advanced ovarian cancer.
OlympiAD is a randomized, open-label, phase III study that evaluated the efficacy and safety of LYNPARZA® (Olaparib) compared with physician’s choice of standard single agent chemotherapy (TPC), in patients with HER2-negative metastatic breast cancer, with inherited, germline BRCA mutations. In this study, 302 patients were randomized in a 2:1 ratio to receive LYNPARZA® tablets 300 mg PO BID (N=205) or physician’s choice of standard chemotherapy (N=97). The later included 21-day cycles of either XELODA® (Capecitabine) 2500 mg/m2 orally on days 1-14, NAVELBINE® (Vinorelbine) 30 mg/m2 IV days 1 and 8 or HALAVEN® (Eribulin)1.4 mg/m2 IV days 1 and 8. Treatment was continued until disease progression or unacceptable toxicity. The median age was 44 years, 50% of the patients had triple negative disease, 71% of the patients had prior chemotherapy for metastatic breast cancer, 28% had prior platinum based chemotherapy regimen and those with hormone receptor positive breast cancer had received hormonal therapy. The primary endpoint was Progression Free Survival (PFS). Secondary endpoints included Overall Survival, time to second progression or death, Objective Response Rate and effect on health-related Quality of Life.
At a median follow up of about 14 months, the median PFS was 7 months in the LYNPARZA® group versus 4.2 months with standard chemotherapy (HR=0.58; P=0.0009), suggesting a 42% reduced risk of cancer progression in the LYNPARZA® group compared to those who received chemotherapy. Following disease progression, the time to second progression (which meant duration of time before the cancer worsened again), was also longer in the LYNPARZA® group (HR 0.57), suggesting that recurrent disease was not more aggressive following progression on LYNPARZA®. The Objective Response Rate was 60% and 29% in LYNPARZA® and chemotherapy group respectively. Severe side effects were more common in chemotherapy treated patients (50%) compared with LYNPARZA® group (37%). The most common side effects in the LYNPARZA® group included nausea, fatigue and cytopenias, where as rash on hands and feet were most common in the chemotherapy group.
The authors concluded that LYNPARZA® monotherapy significantly improved Progression Free Survival in HER2-negative metastatic breast cancer patients, with inherited germline BRCA mutations, compared to standard chemotherapy. This “proof of the principle” study demonstrated that breast cancers with defects in a specific DNA damage repair pathway are sensitive to targeted therapy and this is the first of several phase III studies with PARP inhibitors that are underway. OlympiAD: Phase III trial of olaparib monotherapy versus chemotherapy for patients (pts) with HER2-negative metastatic breast cancer (mBC) and a germline BRCA mutation (gBRCAm). Robson ME, Im S-A, Senkus E, et al. J Clin Oncol 35, 2017 (suppl; abstr LBA4).
Final Overall Survival Results with KADCYLA® in Metastatic Breast Cancer
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately, 255,180 new cases of invasive breast cancer will be diagnosed in 2017 and over 41,070 women will die of the disease. The HER or erbB family of receptors consist of HER1, HER2, HER3 and HER4. Approximately 15%-20% of invasive breast cancers overexpress HER2/neu oncogene, which is a negative predictor of outcomes without systemic therapy. HERCEPTIN® (Trastuzumab) is a humanized monoclonal antibody targeting HER2. It binds to the extracellular domain of the receptor and blocks the downstream cell signaling pathways (PI3K-AKT pathway) and induces Antibody Dependent Cellular Cytotoxicity (ADCC). HERCEPTIN® in combination with chemotherapy has been proven to significantly improve Progression Free Survival and Overall Survival in patients with advanced breast cancer. Despite this benefit, majority of these patients develop progressive disease within 18 months. The tumors in these patients continue to express HER2 although the lower sensitivity to HER2 targeted agents has been attributed to HER2 independent escape mechanisms. Treatment strategies for this patient population have included switching chemotherapy in subsequent lines of treatment and continuing HERCEPTIN®, combining another HER2 targeted agent, Lapatinib (TYKERB®) with Capecitabine (XELODA®) and dual HER2 inhibition with a combination of HERCEPTIN® and TYKERB®.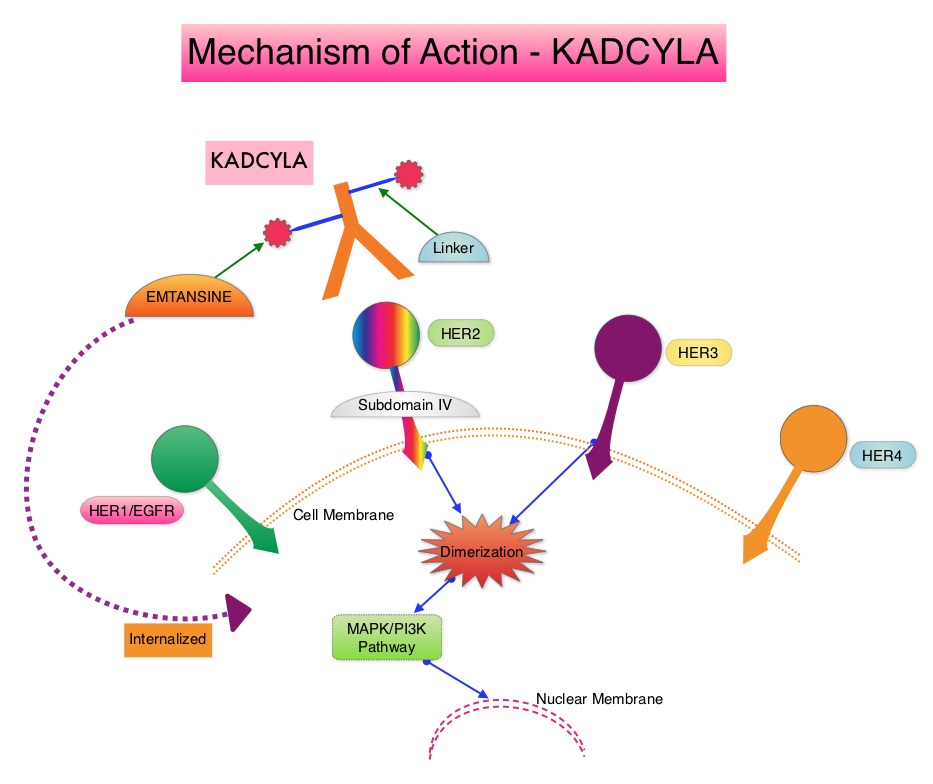
KADCYLA® (Ado-Trastuzumab Emtansine, T-DM1) is an Antibody-Drug Conjugate (ADC) comprised of the antibody HERCEPTIN® and the chemotherapy agent Emtansine, linked together. Upon binding to the HER2 receptor, it not only inhibits the HER2 signaling pathways but also delivers a chemotherapy agent Emtansine, a microtubule inhibitor, directly inside the tumor cells. This agent is internalized by lysosomes and destroys the HER2-positive tumor cells upon intracellular release. In the EMILIA trial, KADCYLA® was associated with significant increase in Overall Survival when compared with TYKERB® plus XELODA®, in HER2-positive metastatic breast cancer patients, who had previously received HERCEPTIN® and a taxane.
TH3RESA is an open label randomized phase III trial in which KADCYLA® was compared with treatment of physician’s choice, in patients with unresectable locally advanced, recurrent or metastatic breast cancer. Eligible patients had a left ventricular ejection fraction of 50% or more and had HER2-positive advanced breast cancer and had received two or more HER2-directed regimens in the advanced setting, and had progressed on both HERCEPTIN® and TYKERB® containing regimens in metastatic setting, and also had disease progression on a taxane, in any setting. Patients were randomized in a 2:1 ratio to receive either KADCYLA® 3.6 mg/kg IV every 21 days (N=404) or treatment of physician’s choice (N=198), which included HER2 directed therapy for the majority of patients. Treatment was continued until disease progression or unmanageable toxicity. The Co-primary endpoints were Progression Free Survival (PFS) and Overall Survival (OS). Secondary endpoints included Response Rates, Duration of Response, Safety and Quality of Life. The authors had previously reported a significant improvement in PFS with KADCYLA® compared with physician's treatment choice (6.2 months vs 3.3 months, HR= 0.528, P<0.0001) and an OS trend favoring KADCYLA®.
The authors now reported the results from the final Overall Survival analysis of the TH3RESA trial. At data cutoff, 47% of the patients in the physician's choice group had crossed over to KADCYLA®. The Overall Survival was significantly longer with KADCYLA® compared with treatment of physician's choice (median 22.7 months versus 15.8 months, HR=0.68, P=0.0007). This benefit was seen in all pre-specified subgroups. Patients in the KADCYLA® group had a lower incidence of grade 3 toxicities compared to the patients in the physician’s treatment choice group (40% vs 47%). Grade 3 thrombocytopenia however was more common in the KADCYLA® group compared to the physician’s choice group (6% vs 3%) and this has been attributed to the inhibition of megakaryocyte differentiation by KADCYLA®.
The authors concluded that for patients who had progressed on two or more HER2-directed regimens, treatment with KADCYLA® significantly improved Overall Survival, compared with treatment of physician's choice, thereby validating HER2 as a therapeutic target, even after multiple lines of previous therapy. Trastuzumab emtansine versus treatment of physician's choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. Krop IE, Kim SB, González-Martín A, et al. Lancet Oncol. DOI: http://dx.doi.org/10.1016/S1470-2045(17)30313-3
