SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately 266,120 new cases of invasive breast cancer will be diagnosed in 2018 and about 40,920 women will die of the disease. The HER or erbB family of receptors consist of HER1, HER2, HER3 and HER4. Approximately 15-20% of invasive breast cancers overexpress HER2/neu oncogene, which is a negative predictor of outcomes without systemic therapy. HERCEPTIN® (Trastuzumab) is a humanized monoclonal antibody targeting HER2, and adjuvant chemotherapy given along with HERCEPTIN® reduces the risk of disease recurrence and death, among patients with HER2-positive, early stage breast cancer. The duration of adjuvant HERCEPTIN® therapy has been 12 months and this length of treatment was empirically adopted from the pivotal registration trials.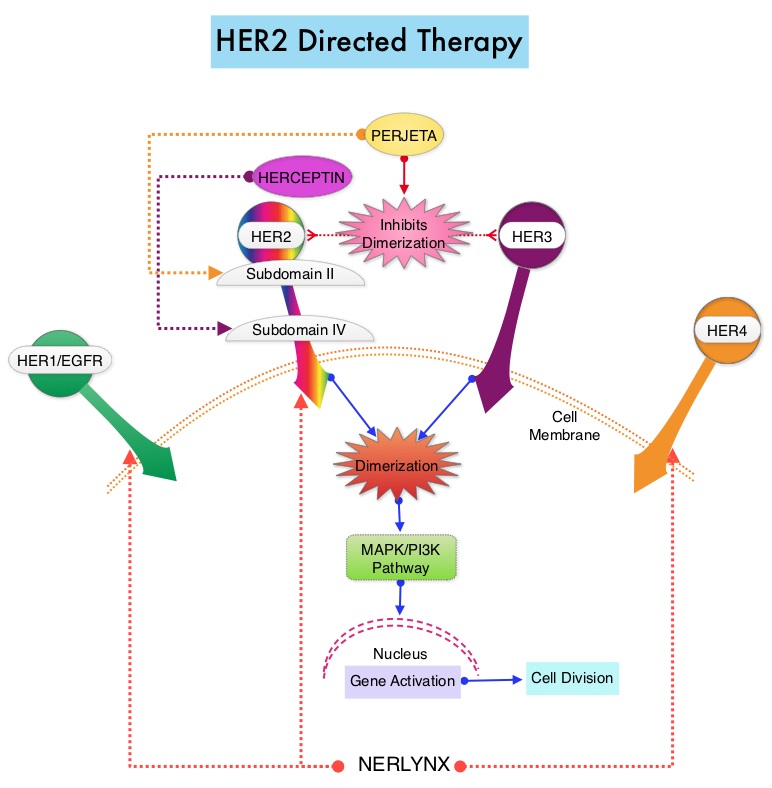
PERSEPHONE is a randomized, phase III, noninferiority trial in which a 6-month course of adjuvant HERCEPTIN® was compared with the standard 12-month course, among patients with HER2-positive early breast cancer. This study was conducted based on the hypothesis that shorter course of treatment with HERCEPTIN® could reduce cardiotoxicities as well as cost without compromising efficacy. This trial randomized 4089 patients across 152 sites in a 1:1 ratio to receive HERCEPTIN® for 6 months (N=2044) or 12 months (N=2045). In this trial, 69% of patients had ER-positive tumors, 41% received Anthracycline-based chemotherapy, 49% received Anthracycline and Taxane-based chemotherapy, 10% received Taxane-based chemotherapy, 85% received adjuvant chemotherapy, and sequential HERCEPTIN® was administered in 54% of patients. This study also included assessment of Left Ventricular Ejection Fraction (LVEF) every 3 months until month 12, as well as continued Quality of Life and health economic assessments at months 18 and 24. The Primary endpoint was Disease Free Survival (DFS) from the time of diagnosis.
At a median follow-up period of 5 years, the 4-year DFS rate was identical in both treatment groups. DFS was 89.8% with 12 months of HERCEPTIN® compared with 89.4% with the 6-month course, which met the criteria for noninferiority (P=0.01). Further, only 4% of the patients enrolled in the 6-month HERCEPTIN® group discontinued HERCEPTIN® treatment due to cardiotoxicities compared with 8% in the 12-month group (P<0.0001), suggesting that the number of patients stopping treatment due to cardiac toxicities was cut in half with the shorter duration of treatment with HERCEPTIN®. Patients receiving shorter course of HERCEPTIN® also had a more rapid recovery of their cardiac LVEF following treatment, compared with the standard of care group (P=0.02).
It was concluded from this largest, reduced duration, noninferiority trial that a shorter 6-month course of adjuvant HERCEPTIN® was noninferior for Disease Free Survival, compared with the standard 12-month schedule, among patients with HER2-positive early breast cancer, with an additional benefit of reduction in cardiac toxicities, as well as cost both to the patients and healthcare systems. Overall Survival data was not available at the time of this analysis. PERSEPHONE: 6 versus 12 months (m) of adjuvant trastuzumab in patients (pts) with HER2 positive (+) early breast cancer (EBC): Randomised phase 3 non-inferiority trial with definitive 4-year (yr) disease-free survival (DFS) results. Earl HM, Hiller L, Vallier A-L, et al. J Clin Oncol 36, 2018 (suppl; abstr 506)

