SUMMARY: The FDA on December 20, 2017, granted regular approval to the anti-PD1 monoclonal antibody, OPDIVO® (Nivolumab) for the adjuvant treatment of patients with melanoma with involvement of lymph nodes or in patients with metastatic disease who have undergone complete resection. OPDIVO® was previously approved for the treatment of patients with unresectable or metastatic melanoma. It is estimated that in the US, approximately 91,270 new cases of melanoma will be diagnosed in 2018 and about 9,320 patients are expected to die of the disease. The incidence of melanoma has been on the rise for the past three decades. Stage III malignant melanoma is a heterogeneous disease and the risk of recurrence is dependent on the number of positive nodes as well as presence of palpable versus microscopic nodal disease. Further, patients with a metastatic focus of more than 1 mm in greatest dimension in the affected lymph node, have a significantly higher risk of recurrence or death than those with a metastasis of 1 mm or less. Patients with stage IIIA disease have a disease-specific survival rate of 78%, whereas those with stage IIIB and stage IIIC disease have a disease specific survival rates of 59% and 40% respectively.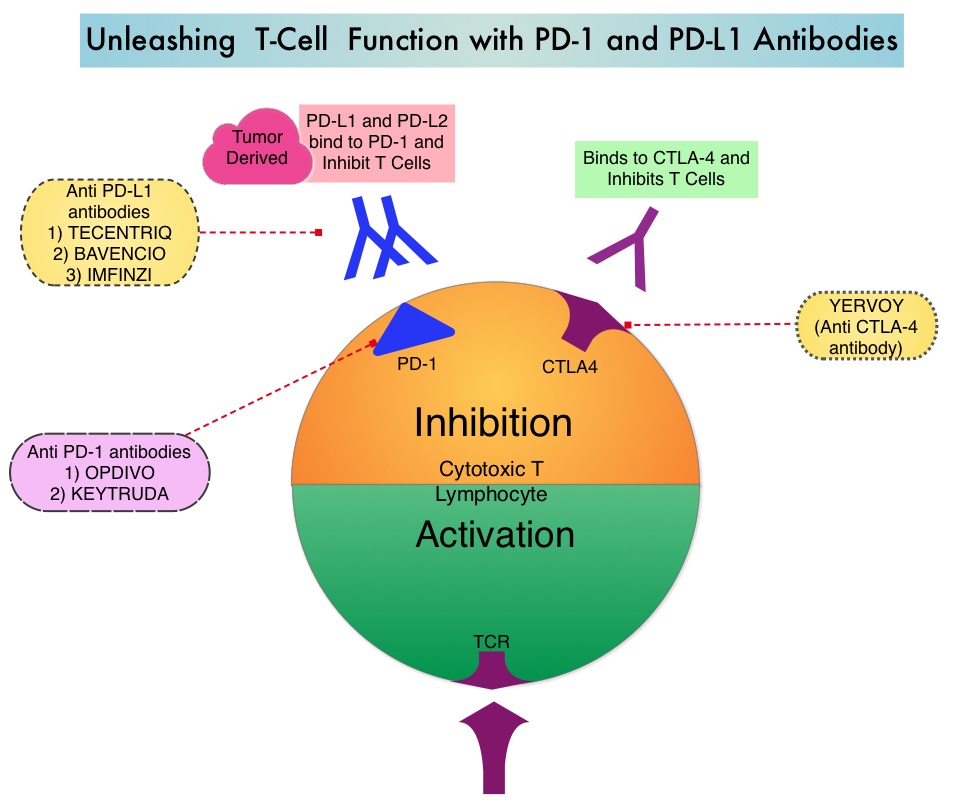
Immune checkpoints are cell surface inhibitory proteins/receptors that harness the immune system and prevent uncontrolled immune reactions. Immune checkpoint proteins (“gate keepers”) suppress antitumor immunity. Antibodies targeting these, membrane bound, inhibitory, Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), etc., block the Immune checkpoint proteins and unleash T cells, resulting in T cell proliferation, activation and a therapeutic response.
The approved adjuvant therapies over the past two decades, for patients with high-risk melanoma have included high-dose INTRON® A (Interferon alfa-2b), SYLATRON® (peginterferon alfa-2b), and high-dose YERVOY® (Ipilimumab). The significant toxicities associated with these adjuvant interventions, precluded the wide spread use of adjuvant therapy in high risk melanoma. YERVOY® was approved by the FDA for the adjuvant treatment of patients with completely resected, Stage III melanoma, based on an improvement in Relapse Free Survival, when compared to placebo, in a randomized phase III trial. In this study however, over 50% of the patients treated with the recommended high dose YERVOY® experienced grade 3/4 toxicities. There is therefore an unmet need for adjuvant therapies, with improved benefit-risk ratio, for this patient group.
OPDIVO® (Nivolumab) is a fully human, immunoglobulin G4 monoclonal antibody that targets PD-1 receptor. Monotherapy with OPDIVO®, in heavily pretreated advanced Melanoma patients can result in more than a third of patients (34%) being alive, 5 years after starting treatment. The present approval by the FDA was based on CheckMate 238 trial, which is a double-blind, phase III study that included 906 patients with completely resected, Stage IIIB/C or Stage IV melanoma. Patients were randomized in a 1:1 ratio to receive either OPDIVO® 3 mg/kg IV, every 2 weeks (N=453) or YERVOY® 10 mg/kg IV, every 3 weeks (N=453) for 4 doses, then every 12 weeks beginning at week 24, for up to 1 year. Both treatment groups were well balanced. The Primary end point was Recurrence Free Survival (RFS).
The Recurrence Free Survival rate at 18 months with OPDIVO® was 66.4% compared with 52.7% for YERVOY® (HR=0.65; P<0.0001). This meant a 35% reduction in the risk of recurrence or death with the OPDIVO® versus YERVOY®. The most common adverse reactions were headache, fatigue, nausea, diarrhea, abdominal discomfort, rash, pruritus and musculoskeletal pain. OPDIVO® was associated with significantly fewer treatment-related grade 3/4 toxicities compared to YERVOY® (14% versus 46%). Treatment was discontinued due to toxicities in approximately 10% of the patients in the OPDIVO® group, compared with 42% of patients in the YERVOY® group.
The authors concluded that OPDIVO® is a less toxic, better tolerated, adjuvant treatment option than YERVOY®, for patients with resected stage IIIB/C and IV melanoma, regardless of BRAF mutation, with a superior Relapse Free Survival rate. Weber J, Mandala M, Del Vecchio M, et al. Adjuvant therapy with nivolumab (NIVO) versus ipilimumab (IPI) after complete resection of stage III/IV melanoma: a randomized, double-blind, phase 3 trial (CheckMate 238). Presented at ESMO 2017 Congress; September 8-12, 2017; Madrid, Spain. Abstract LBA8_PR.

