SUMMARY: Cancer mortality rates in the United States have declined 20% from their peak of 215 per 100,000 in 1991 to 172 per 100,000 in 2010. Part II of this Annual Report on Progress Against Cancer explores, ADVANCES IN TREATMENT, ADVANCES IN TUMOR BIOLOGY AND ADVANCES IN PATIENT CARE. Clinical study details for several of these studies can be accessed at www.oncoprescribe.com
ADVANCES IN TREATMENT
COMBINATION THERAPY
Chemotherapy and Radiotherapy significantly improves Survival for Patients with Low-Grade Glioma – Radiotherapy has been the standard first-line treatment for patients with low-grade glioma. In a study involving 251 patients with gliomas which included grade 2 Astrocytoma, Oligoastrocytoma, or Oligodendroglioma, the addition of chemotherapy (PCV regimen – (Procarbazine, Lomustine, and Vincristine) to radiation extended median survival by 5.5 years (13.3 vs 7.8 years, P=0.03; HR=0.59) and also resulted in a longer median Progression Free survival (10.4 vs 4 years, P=0.002; HR=0.50), when compared with radiotherapy alone.
First-Line Chemotherapy Added to Hormone Therapy Improves Survival for Men With Advanced Prostate Cancer – Androgen Deprivation Therapy (ADT) has been the cornerstone of treatment of advanced prostate cancer and is the first treatment intervention for hormone sensitive prostate cancer. Chemotherapy is usually considered for patients who progress on hormone therapy. In a pivotal phase III study which included 790 men with metastatic, hormone-sensitive prostate cancer, the addition of TAXOTERE® (Docetaxel) chemotherapy to ADT improved median Overall Survival by 10 months from 42.3 months in the ADT alone group to 52.7 months in the ADT plus TAXOTERE® group (HR=0.63; P<0.0006). The median time to clinical progression was 19.8 months in the ADT alone group vs 32.7 months in the ADT plus TAXOTERE® group (P < 0.0001).
TARGETED THERAPY
Overcoming Resistance to EGFR Targeted Agents in Lung Cancer– TARCEVA® (Erlotinib) and GILOTRIF® (Afatinib) are recommended as first-line treatments for patients harboring Epidermal Growth Factor Receptor (EGFR) gene mutations, which are detected in 15% of Caucasian and 40% of Asian patients with NSCLC. An additional EGFR mutation (T790M) is responsible for resistance to EGFR-targeted therapy and may be detected in about 50% of those harboring EGFR mutations in Non Small Cell Lung Cancer (NSCLC). AZD9291 and CO-1686 are two new agents which have demonstrated a 50-60% response rate in patients with T790M mutation.
New second-line treatment options for ALK-positive NSCLC – XALKORI® (Crizotinib) significantly improves PFS and Response Rates in patients with ALK-positive NSCLC. Approximately one third of these patients are resistant to XALKORI® and this has been attributed to acquired mutation within the ALK tyrosine kinase domain, amplification of the ALK fusion gene, subtherapeutic inhibition of ALK tyrosine kinase or activation of other pathways that can cause abnormal cell proliferation. ZYKADIA® (Ceritinib) resulted in an overall Response Rate (RR) of 58% and median PFS of 7 months in this patient population, with responses seen in untreated CNS lesions as well.
FDA Approves First Treatment for Chemotherapy-Resistant, Advanced Stomach Cancer – CYRAMZA® (Ramucirumab) an angiogenesis inhibitor was approved for the treatment of advanced stomach cancer or gastroesophageal junction adenocarcinoma that progressed during or after chemotherapy. The approval was based on a phase III trial involving 355 patients in which patients treated with CYRAMZA® (Ramucirumab) had longer survival (5.2 vs 3.8 months, HR = 0.78, P=0.047), when compared to placebo.
Lenvatinib: A New Option for a Difficult-to-Treat Thyroid Cancer – LENVIMA® (Lenvatinib) a Tyrosine Kinase Inhibitor, was approved for the treatment of patients with locally recurrent or metastatic, progressive, RadioActive Iodine (RAI)-refractory Differentiated Thyroid Cancer (DTC). In a phase III study involving 392 patients with advanced RAI-refractory Differentiated Thyroid Cancer (DTC), the median Progression Free Survival was 18.3 months in the LENVIMA® group and 3.6 months in the placebo group (HR= 0.21; P<0.001). The objective response rate with LENVIMA® was 64.8% versus 1.5% with placebo (P<0.001). Another targeted drug, NEXAVAR® (Sorafenib), was approved for the same patient population in 2013.
IMMUNOTHERAPY
Both antibody and cell-based immunotherapy approaches have taken center stage in cancer immunotherapy. Check point inhibitors such as anti CTLA-4. Anti PD-1 and anti PDL1 antibodies unleash the immune system allowing the T cells to attack the malignant cells.
Adjuvant Immunotherapy For Early Stage Melanoma – YERVOY® (Ipilimumab), an anti CTLA-4 antibody decreased the relative risk of recurrence by 25% (HR=0.75; P=0.0013), when compared with placebo, in patients with high risk, completely resected, stage III melanoma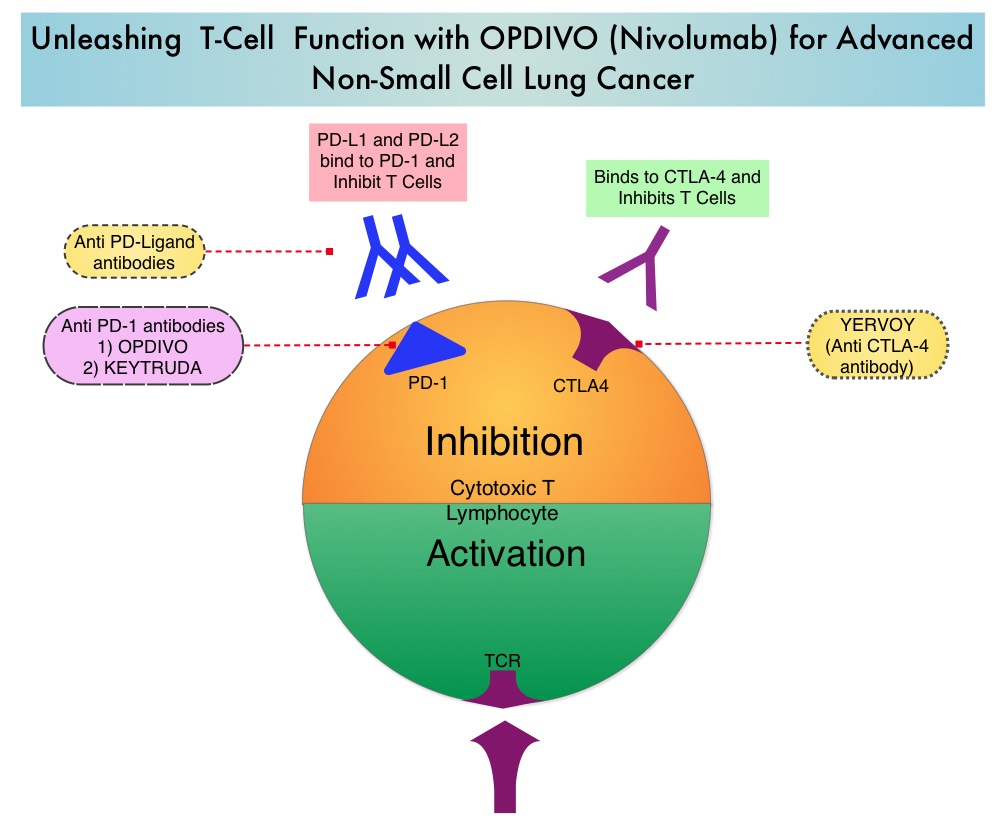
Immunotherapy in Lung Cancer – OPDIVO® (Nivolumab), a PD-1 targeted monoclonal antibody improved overall survival by 41% (HR=0.59; P=0.00025) when compared to TAXOTERE® (Docetaxel) in a randomized phase III trial in patients with metastatic squamous NSCLC, who had experienced disease progression during or after one prior platinum-based chemotherapy regimen. KEYTRUDA® (Pembrolizumab) another PD-1 antibody demonstrated superior responses in patients with lung cancer when the tumors were PD-L1 positive versus PD-L1 negative (23% vs 9%) suggesting that PD-L1 may be a predictor of response to PD-1 and PD-L1 therapies.
Tumor Directed Chimeric Antigen Receptor (CAR) T-Cell Therapy – In this type of immunotherapy, T cells are collected from the patient’s own blood and are genetically engineered to produce special receptors on their surface called chimeric antigen receptors (CAR’s). These cytotoxic T cells with these chimeric antigen receptors on their surface are now able to recognize a specific antigen on tumor cells. These engineered CAR T-cells (CTL019) which are grown in vitro, are then infused into the patient and they in turn proliferate in the patient’s body, recognize and kill cancer cells expressing that specific antigen. In a small study of patients with relapsed or refractory ALL, treatment with autologous Chimeric Antigen Receptor (CAR) T-cells (CTL019 T-cells) resulted in a 90% remission rate with sustained remissions for up to 2 years and overall survival of 78%.
ADVANCES IN TUMOR BIOLOGY
Genomic Profile Based Therapy – In a recent landmark study, analysis of molecular data from approximately 3,500 patients with 12 different forms of cancer, lead to the identification of 11 major molecular subtypes. It was noted that most malignancies that originated from the same tissue or organ had similar genomic profiles. Treatment in the foreseeable future may be based on genomic profile rather than site of origin of the malignancy.
Blood Test Predicts Resistance to Prostate Cancer Therapy – In patients with metastatic CRPC, the presence of Androgen Receptor Variant AR-V7 rather than a normal androgen receptor, in the circulating tumor cells (CTCs) before, during, and after treatment with either XTANDI® (Enzalutamide) or ZYTIGA® (Abiraterone), conferred resistance to these agents. The Androgen Receptor Variant AR-V7 was detected in roughly 40% of patients treated with XTANDI® and 20% of those treated with ZYTIGA®.
Gut Bacteria and Response to Therapy – Two early studies have demonstrated that Intestinal bacteria may be beneficial in priming and mobilizing immune cells to attack tumors. Further, certain chemotherapeutic agents such as CYTOXAN® (Cyclophosphamide) and ELOXATIN® (Oxaliplatin) were less effective when intestinal bacteria were eradicated with antibiotics.
ADVANCES IN PATIENT CARE
Early Initiation of Palliative Care Improves Patient Well Being – In a study of close to 500 patients with advanced cancer early palliative care improved, quality of life at the end of life, spiritual well-being, symptom severity, and satisfaction with care at 4 months after diagnosis.
Pregnancy After Breast Cancer Treatment – Premature Ovarian Failure (POF) is a common unintended consequence of chemotherapy in premenopausal women. Besides loss of fertility, which can influence treatment decisions in young women, ovarian failure can lead to menopausal symptoms, sexual dysfunction and loss of bone density. Two studies reported a promising new way of preserve fertility. In the POEMS (Prevention of Early Menopause Study) phase III trial, the addition of ZOLADEX® (Goserelin) to chemotherapy decreased the POF to 8% compared to 22% with chemotherapy alone (P=0.04). More women in the ZOLADEX® group achieved at least one pregnancy (21%) compared to 11% in the chemotherapy alone group (P=0.03). In a different study, similar findings were noted with the addition of another hormonal agent, TRELSTAR® (Triptorelin) to chemotherapy.
Masters GA, Krilov L, Bailey HH , et al. Published online before print January 20, 2015, doi: 10.1200/JCO.2014.59.9746

