SUMMARY: The Center for Disease Control and Prevention (CDC) estimates that approximately 1-2 per 1000 individuals develop Deep Vein Thrombosis/Pulmonary Embolism (PE) each year in the United States, resulting in 60,000 – 100,000 deaths. Venous ThromboEmbolism (VTE) is the third leading cause of cardiovascular mortality. Patients with unprovoked DVT and PE are two to four times more likely to be diagnosed with cancer within the following 12 months compared to the general population. In patients with cancer associated thrombosis, COUMADIN® (Warfarin) and XARELTO® (Rivaroxaban) are often prescribed, despite guidelines recommending Low Molecular Weight Heparin (LMWH) in this patient population.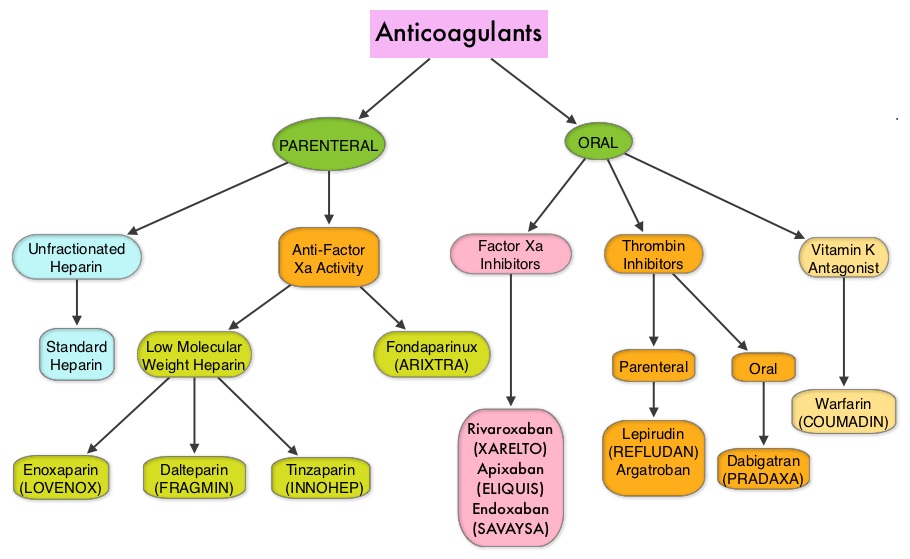
Recently published data suggests that the rates of major bleeding, with use of XARELTO® in a highly selected group of cancer patients with venous thromboembolic disease, compared favorably with those treated with LMWH. (Mantha S, et al. 2015 ASH Annual Meeting). There is however limited data comparing the efficacy of different anticoagulants for VTE treatment in cancer patients.
The authors conducted this study in cancer patients, to compare the VTE recurrence rates, following most frequently prescribed anticoagulants in the United States. Newly diagnosed cancer patients with a first VTE, who initiated LMWH, COUMADIN® or XARELTO®, were selected using healthcare claims from the Humana database. The study population included 2,428 patients (XARELTO®: N=707; LMWH: N=660; COUMADIN® N =1,061). VTE recurrences were defined as hospitalizations with a primary diagnosis of VTE. Outpatients with a primary diagnosis of VTE were added as a sensitivity analysis to the recurrence definition.
The median duration on initial LMWH treatment was 1 month, on COUMADIN® was 3.5 months and on XARELTO® was 3 months. When compared to LMWH, VTE recurrence rates were lower with initial XARELTO® treatment at 6 months (13.2% versus 17.1%; P=0.06) and at 12 months (16.5% versus 22.2%; P=0.03). When initially treated with XARELTO®, recurrent VTE was 28% less likely than with LMWH (HR=0.72; P<0.03).
When compared to COUMADIN®, VTE recurrence rates were again lower with initial XARELTO® treatment at 6 months (13.2% versus 17.5%; P=0.02) and at 12 months (15.7% versus 19.9%; P=0.02). When initially treated with XARELTO®, recurrent VTE was 26% less likely than with COUMADIN® (HR=0.74; P<0.03). This benefit with XARELTO® when compared with LMWH and COUMADIN® users, was also noted in the sensitivity analysis.
The authors concluded that based on this real world healthcare claims data in cancer patients, XARELTO® was associated with a lower risk of recurrent VTE than LMWH or COUMADIN® and this could be a reflection of a shorter duration of treatment with LMWH and difficult therapeutic anticoagulation with COUMADIN®. Recurrent VTE in cancer patients treated with anticoagulation. Streiff MB, Milentijevic D, McCrae K, et al. J Clin Oncol 34, 2016 (suppl; abstr 10024)

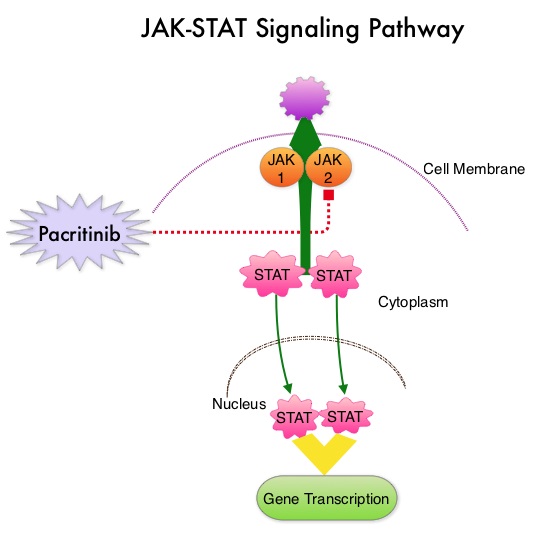 The JAK-STAT signaling pathway has been implicated in the pathogenesis of Myelofibrosis. This pathway normally is responsible for passing information from outside the cell through the cell membrane to the DNA in the nucleus, for gene transcription. Janus Kinase (JAK) family of tyrosine kinases are cytoplasmic proteins and include JAK1, JAK2, JAK3 and TYK2. JAK1 helps propagate the signaling of inflammatory cytokines whereas JAK2 is essential for growth and differentiation of hematopoietic stem cells. These tyrosine kinases mediate cell signaling by recruiting STAT’s (Signal Transducer and Activator of Transcription), with resulting modulation of gene expression. In patients with MPN, the aberrant myeloproliferation is the result of dysregulated JAK2-STAT signaling as well as excess production of inflammatory cytokines associated with this abnormal signaling. These cytokines contribute to the symptoms often reported by patients with MF. JAK2 mutations such as JAK2 V617F are seen in approximately 60% of the patients with PMF and ET and 95% of patients with PV. Unlike CML where the BCR-ABL fusion gene triggers the disease, JAK2 mutations are not initiators of the disease and are not specific for MPN. Further, several other genetic events may contribute to the abnormal JAK2-STAT signaling.
The JAK-STAT signaling pathway has been implicated in the pathogenesis of Myelofibrosis. This pathway normally is responsible for passing information from outside the cell through the cell membrane to the DNA in the nucleus, for gene transcription. Janus Kinase (JAK) family of tyrosine kinases are cytoplasmic proteins and include JAK1, JAK2, JAK3 and TYK2. JAK1 helps propagate the signaling of inflammatory cytokines whereas JAK2 is essential for growth and differentiation of hematopoietic stem cells. These tyrosine kinases mediate cell signaling by recruiting STAT’s (Signal Transducer and Activator of Transcription), with resulting modulation of gene expression. In patients with MPN, the aberrant myeloproliferation is the result of dysregulated JAK2-STAT signaling as well as excess production of inflammatory cytokines associated with this abnormal signaling. These cytokines contribute to the symptoms often reported by patients with MF. JAK2 mutations such as JAK2 V617F are seen in approximately 60% of the patients with PMF and ET and 95% of patients with PV. Unlike CML where the BCR-ABL fusion gene triggers the disease, JAK2 mutations are not initiators of the disease and are not specific for MPN. Further, several other genetic events may contribute to the abnormal JAK2-STAT signaling.
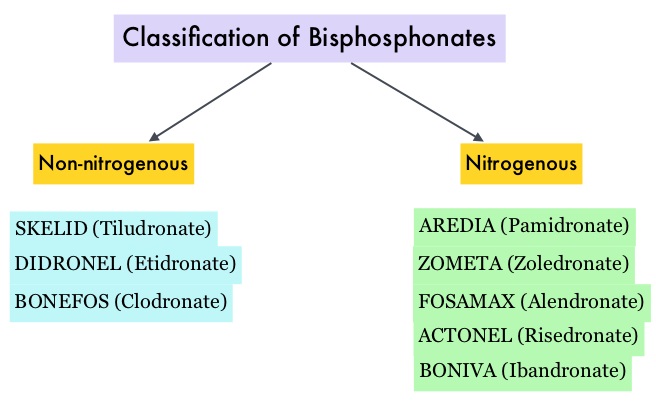 Bisphosphonates can also reduce bone pain and may improve Quality of life. Intravenous bisphosphonates, Pamidronate (AREDIA®) and Zoledronic acid (ZOMETA®) have been approved in the US for the treatment of bone metastases. Amino-bisphosphonate, ZOMETA® has however largely replaced AREDIA®, because of its superior efficacy. Both AREDIA® and ZOMETA® are administered IV every 3 to 4 weeks during the first year, following diagnoses of bone metastases. However, the optimal treatment schedule following this initial phase of treatment has remained unclear. Further, renal toxicity, long bone fractures and OsteoNecrosis of the Jaw (ONJ) have been identified as potential problems with bisphosphonate use.
Bisphosphonates can also reduce bone pain and may improve Quality of life. Intravenous bisphosphonates, Pamidronate (AREDIA®) and Zoledronic acid (ZOMETA®) have been approved in the US for the treatment of bone metastases. Amino-bisphosphonate, ZOMETA® has however largely replaced AREDIA®, because of its superior efficacy. Both AREDIA® and ZOMETA® are administered IV every 3 to 4 weeks during the first year, following diagnoses of bone metastases. However, the optimal treatment schedule following this initial phase of treatment has remained unclear. Further, renal toxicity, long bone fractures and OsteoNecrosis of the Jaw (ONJ) have been identified as potential problems with bisphosphonate use.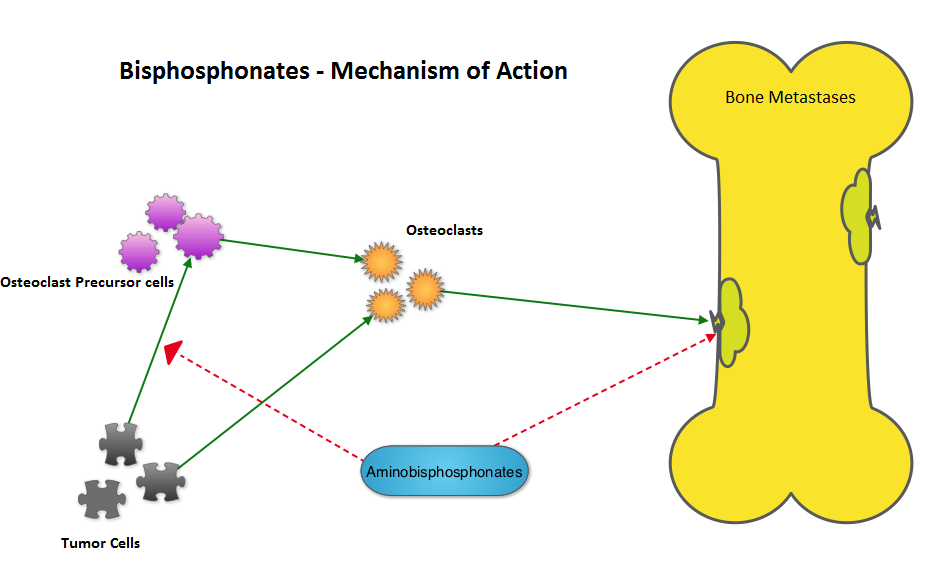 The primary endpoint was incidence of any Skeletal Related Event (SRE) and secondary endpoints included skeletal morbidity rates, performance status, pain using the Brief Pain Inventory and incidences of ONJ and renal dysfunction. Both treatment groups were well matched. Patients in this trial were stratified by disease and analyses by disease was pre-planned. It was noted that for the primary endpoint, there was no significant difference between the two treatment groups with 29% of patients in both treatment groups experiencing at least one SRE (P=0.79). With regards to secondary endpoints, there were still no significant differences between the two treatment groups, including renal dysfunction and ONJ. The authors pointed out that toxicities such as ONJ and renal dysfunction are more likely to occur after 2 years of treatment.
The primary endpoint was incidence of any Skeletal Related Event (SRE) and secondary endpoints included skeletal morbidity rates, performance status, pain using the Brief Pain Inventory and incidences of ONJ and renal dysfunction. Both treatment groups were well matched. Patients in this trial were stratified by disease and analyses by disease was pre-planned. It was noted that for the primary endpoint, there was no significant difference between the two treatment groups with 29% of patients in both treatment groups experiencing at least one SRE (P=0.79). With regards to secondary endpoints, there were still no significant differences between the two treatment groups, including renal dysfunction and ONJ. The authors pointed out that toxicities such as ONJ and renal dysfunction are more likely to occur after 2 years of treatment. Survival of cancer cells in the human body may be to a significant extent, related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4), also known as CD152, PD-1(Programmed cell Death-1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic respons
Survival of cancer cells in the human body may be to a significant extent, related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4), also known as CD152, PD-1(Programmed cell Death-1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic respons