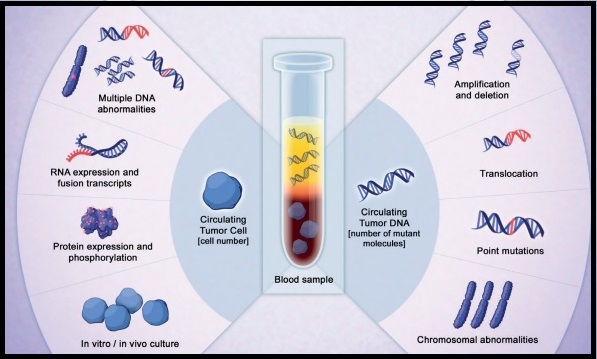
SUMMARY: Myelofibrosis is a MyeloProliferative Neoplasm (MPN) characterized by ineffective hematopoiesis, progressive fibrosis of the bone marrow and potential for leukemic transformation. This stem cell disorder is Philadelphia Chromosome negative and manifestations include anemia, splenomegaly and related symptoms such as abdominal distension and discomfort with early satiety. Cytokine driven debilitating symptoms such as fatigue, fever, night sweats, weight loss, pruritus and bone or muscle pain can further impact an individual’s quality of life. Myelofibrosis can be primary (PMF) or secondary to Polycythemia Vera (PV) or Essential Thrombocythemia (ET). The JAK-STAT signaling pathway has been implicated in the pathogenesis of Myelofibrosis. This pathway normally is responsible for passing information from outside the cell through the cell membrane to the DNA in the nucleus, for gene transcription. Janus Kinase (JAK) family of tyrosine kinases are cytoplasmic proteins and include JAK1, JAK2, JAK3 and TYK2. JAK1 helps propagate the signaling of inflammatory cytokines whereas JAK2 is essential for growth and differentiation of hematopoietic stem cells. These tyrosine kinases mediate cell signaling by recruiting STAT’s (Signal Transducer and Activator of Transcription), with resulting modulation of gene expression. In patients with MPN, the aberrant myeloproliferation is the result of dysregulated JAK2-STAT signaling as well as excess production of inflammatory cytokines associated with this abnormal signaling. These cytokines contribute to the symptoms often reported by patients with MF. JAK2 mutations such as JAK2 V617F are seen in approximately 60% of the patients with PMF and ET and 95% of patients with PV. Unlike CML where the BCR-ABL fusion gene triggers the disease, JAK2 mutations are not initiators of the disease and are not specific for MPN. Further, several other genetic events may contribute to the abnormal JAK2-STAT signaling.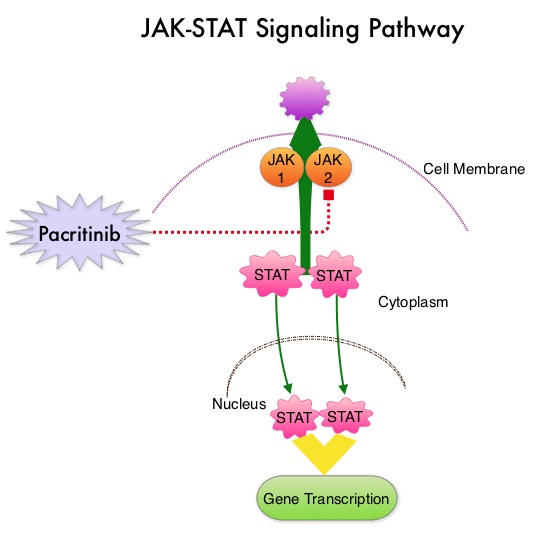
Pacritinib is a potent JAK2 inhibitor, without significant JAK1 inhibition. It additionally targets FLT3, IRAK1, and CSF1R. Preliminary studies have shown minimal myelosuppression with Pacritinib. 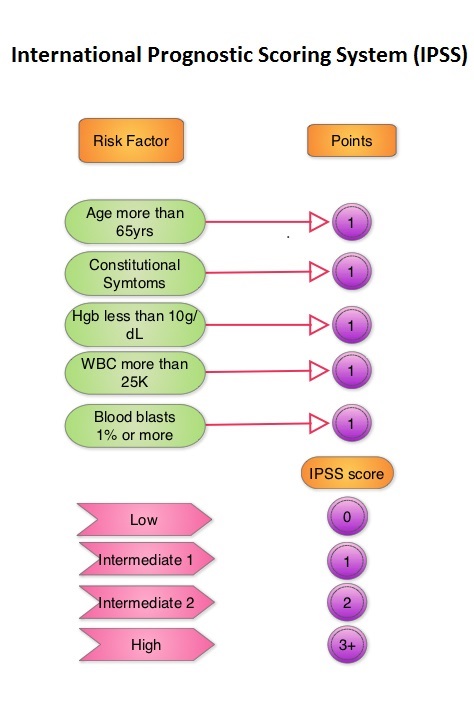 JAKAFI® (Ruxolitinib) is a potent JAK1 and JAK2 inhibitor approved by the FDA in 2011 to treat intermediate or high-risk Myelofibrosis. It is however not indicated for patients with platelet counts under 50,000/μl, and this group represents approximately one third of Myelofibrosis patients and have limited or no treatment options. Previously published PERSIST-1 trial showed that Pacritinib significantly reduced Spleen Volume and Myelofibrosis associated symptoms, in patients with low platelet count, when compared to Best Available Therapy (excluding JAKAFI®).
JAKAFI® (Ruxolitinib) is a potent JAK1 and JAK2 inhibitor approved by the FDA in 2011 to treat intermediate or high-risk Myelofibrosis. It is however not indicated for patients with platelet counts under 50,000/μl, and this group represents approximately one third of Myelofibrosis patients and have limited or no treatment options. Previously published PERSIST-1 trial showed that Pacritinib significantly reduced Spleen Volume and Myelofibrosis associated symptoms, in patients with low platelet count, when compared to Best Available Therapy (excluding JAKAFI®).
PERSIST-2 is an open label, phase III study in which the safety and efficacy of Pacritinib was compared with currently available therapies, including JAKAFI®, thus expanding the definition of Best Available Therapy (BAT). A total of 311 patients with platelet counts 100,000/μl or less were randomly assigned in a 1:1:1 ratio to receive Pacritinib 200 mg BID (N=107), 400 mg QD (N=104) or Best Available Therapy (N=100). The efficacy population in the Intent To Treat group included a total of 221 patients. Approximately half of the study population had platelet counts of less than 50,000/μl. Over 40% of the patients in both the treatment groups had prior therapy with JAKAFI®. About 60-70% of the patients had a diagnoses of Primary Myelofibrosis, and half of the patients fell in the International Prognostic Scoring System (IPSS) Intermediate-2 risk category. The two coprimary endpoints were the proportion of patients achieving 35% or greater reduction in Spleen Volume (SVR) as measured by MRI or CT scan and the proportion achieving a 50% or more improvement in symptoms such as fatigue, bone pain, itching, and abdominal pain after 24 weeks of follow up. The secondary objectives were to compare Pacritinib BID and Pacritinib QD, individually to BAT.
It was noted that 18% of patients who received Pacritinib achieved a 35% or greater reduction in Spleen Volume from baseline to week 24, compared to 3% of those in the BAT group (P=0.001). In the patient group who received Pacritinib twice daily, 32% reported a 50% or more reduction in symptoms compared with 14% in the BAT group (P=0.01). Further, patients treated with Pacritinib required fewer red blood cell transfusions and additionally, patients who received Pacritinib twice daily had substantially greater improvement in platelet count among those who had platelets counts 50,000/μl or less at enrollment. The most common adverse events related to Pacritinib included nausea, vomiting, diarrhea, anemia, and low platelets.
The authors concluded that this is the only randomized trial to date in patients with Myelofibrosis and thrombocytopenia that enrolled patients who had prior therapy with a JAK2 inhibitor. Regardless, Pacritinib was more effective at Spleen Volume Reduction than BAT and Pacritinib given BID was even more effective than QD dosing. Results of the Persist-2 Phase 3 Study of Pacritinib (PAC) Versus Best Available Therapy (BAT), Including Ruxolitinib (RUX), in Patients (pts) with Myelofibrosis (MF) and Platelet Counts <100,000/µl. Hoffman R, Talpaz M, Gerds AT, et al. 58th ASH Annual Meeting and Exposition; San Diego, California; December 2-6, 2016. Abstract LBA-5.

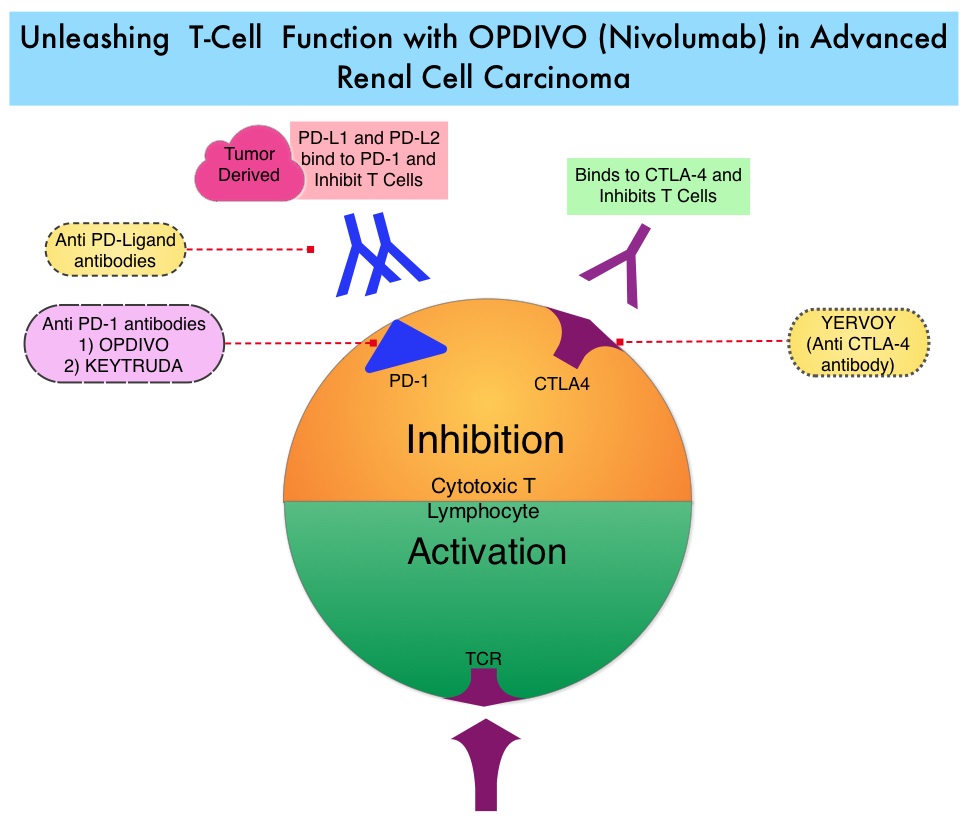
 In the phase II study, 167 patients with advanced Renal Cell Carcinoma with KPS of 70% or more and who had prior treatment with 1-3 regimens in the metastatic setting, received OPDIVO® at a dose of 0.3, 2, or 10 mg/kg every 3 weeks (J Clin Oncol 2015;33:1430–37). At a minimum follow up of 38 months, ORR was 21% and the median Duration of Response was 22 months. The 3 year OS rate was 35% and 4 year OS rate was 29%. Long-term survival was observed in MSKCC good, intermediate and poor-risk patients, as well as in patients with excellent or reduced Karnofsky Performance Status.
In the phase II study, 167 patients with advanced Renal Cell Carcinoma with KPS of 70% or more and who had prior treatment with 1-3 regimens in the metastatic setting, received OPDIVO® at a dose of 0.3, 2, or 10 mg/kg every 3 weeks (J Clin Oncol 2015;33:1430–37). At a minimum follow up of 38 months, ORR was 21% and the median Duration of Response was 22 months. The 3 year OS rate was 35% and 4 year OS rate was 29%. Long-term survival was observed in MSKCC good, intermediate and poor-risk patients, as well as in patients with excellent or reduced Karnofsky Performance Status. The Affordable Care Act in 2010 created an abbreviated licensure pathway for biological products that are demonstrated to be “Biosimilar” to, or “interchangeable” with an FDA-licensed (FDA approved) biological product (reference product). The Biosimilar must show that it has no clinically meaningful differences in terms of safety and effectiveness from the reference product. A Biosimilar product can only be approved by the FDA if it has the same mechanism of action, route of administration, dosage form and strength as the reference product, and only for the indications and conditions of use that have been approved for the reference product. Biosimilars are not as easy to manufacture as generics (copies of brand name drugs) because of the complexity of the structure of the biologic product and the process used to make a biologic product. The facilities where Biosimilars are manufactured must also meet the FDA’s standards.
The Affordable Care Act in 2010 created an abbreviated licensure pathway for biological products that are demonstrated to be “Biosimilar” to, or “interchangeable” with an FDA-licensed (FDA approved) biological product (reference product). The Biosimilar must show that it has no clinically meaningful differences in terms of safety and effectiveness from the reference product. A Biosimilar product can only be approved by the FDA if it has the same mechanism of action, route of administration, dosage form and strength as the reference product, and only for the indications and conditions of use that have been approved for the reference product. Biosimilars are not as easy to manufacture as generics (copies of brand name drugs) because of the complexity of the structure of the biologic product and the process used to make a biologic product. The facilities where Biosimilars are manufactured must also meet the FDA’s standards.