SUMMARY: The FDA on February 9, 2021, granted regular approval to LIBTAYO® (Cemiplimab-rwlc) for patients with locally advanced Basal Cell Carcinoma (laBCC) previously treated with a HedgeHog pathway Inhibitor (HHI) or for whom a HHI is not appropriate, and granted accelerated approval to LIBTAYO® for patients with metastatic BCC (mBCC) previously treated with a HHI or for whom a HHI is not appropriate.
BCC is the most common type of skin cancer in the U.S., with approximately two million new cases diagnosed every year. Exposure to UltraViolet rays is a significant risk factor. Majority of BCCs are diagnosed early and cured with surgery and radiation. However, a small proportion of tumors can become locally advanced or progress to metastatic disease and can be painful and disfiguring. The primary systemic treatment options for these patients with advanced BCC are oral HedgeHog pathway inhibitors such as ERIVEDGE® (Vismodegib) and ODOMZO® (Sonidegib). There are however no FDA-approved treatment options available, for patients who progress on, or are intolerant to HedgeHog Inhibitors (HHIs).
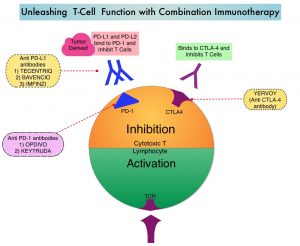
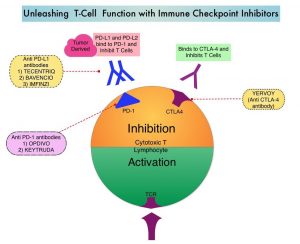
LIBTAYO® is a fully human IgG4, high affinity anti-PD-1, monoclonal antibody, that binds to the PD-1 receptor on tumor-infiltrating T cells and blocks its interaction with tumor derived ligands PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the tumor-specific effector T cells. LIBTAYO® was previously approved by the FDA in 2018 as the first systemic treatment for adults with metastatic Cutaneous Squamous Cell Carcinoma (CSCC) or locally advanced CSCC, who are not candidates for curative surgery or curative radiation.
The present FDA approval of LIBTAYO® was based on results from an interim analysis of an ongoing open-label, multi-center, non-randomized Phase II trial (Study 1620), involving patients with unresectable locally advanced BCC or metastatic BCC (nodal or distant). This was the first and largest prospective clinical trial (N=132) among this patient population, with 112 patients included in the efficacy analysis. Patients in both cohorts (locally advanced and metastatic) had either progressed on HHI therapy, had not had an objective response after 9 months on HHI therapy, or were intolerant of prior HHI therapy. Eligibility required that locally advanced BCC patients were not candidates for curative surgery or curative RT, per multidisciplinary assessment. All patients received LIBTAYO® 350 mg IV over 30 minutes every 3 weeks for up to 93 weeks, until disease progression, unacceptable toxicity, or completion of planned treatment. No PD-L1 or Tumor Mutational Burden (TMB) testing was required before starting treatment with LIBTAYO®. The Primary efficacy endpoint was confirmed Objective Response Rate (ORR) and a key Secondary endpoint was Duration of Response (DOR), as assessed by Independent Central Review.
Among the 84 patients with locally advanced BCC, the confirmed ORR was 29% with a median DOR not reached, and 79% of responders maintained their response for at least 6 months. Among 28 patients with metastatic BCC, the confirmed ORR was 21%, with a median DOR not reached, and all responders maintained their responses for at least 6 months. The most common adverse reactions (incidence 20% or more) were fatigue, musculoskeletal pain, diarrhea, rash, and pruritis.
It was concluded from this study that LIBTAYO® is the first agent to provide clinically meaningful anti-tumor activity including durable responses, in patients with advanced BCC, after progression or intolerance on HHI therapy.
Interim Analysis of Phase 2 Results for Cemiplimab in Patients with Metastatic Basal Cell Carcinoma (mBCC) who Progressed on or are Intolerant to Hedgehog Inhibitors (HHIs). Lewis KD, Peris K, Sekulic A, et al. Presented at the 2021 Winter Clinical Dermatology Conference, January 16–24, Virtual Conference (encore of SITC 2020 poster presentation).