SUMMARY: Multiple Myeloma is a clonal disorder of plasma cells in the bone marrow and the American Cancer Society estimates that in the United States, 24,050 new cases were diagnosed in 2014 and 11,090 died of the disease. KYPROLIS® (Carfilzomib) is a second generation selective, epoxyketone proteasome inhibitor and unlike VELCADE® (Bortezomib), proteasome inhibition with KYPROLIS® is irreversible. KYPROLIS® monotherapy is presently approved in the United States for use in patients with relapsed and refractory Multiple Myeloma following a phase 2b single arm study which showed a 24% overall response rate in this patient group. REVLIMID® (Lenalidomide) given along with weekly Dexamethasone, was associated with significantly improved Progression Free Survival (PFS) when administered until disease progression, in patients with newly diagnosed Multiple Myeloma. The combination of REVLIMID® and weekly Dexamethasone is considered a reference regimen for both newly diagnosed and relapsed multiple myeloma. VELCADE® in combination with REVLIMID® and Dexamethasone showed an overall response rate of 64% and a median PFS of 9.5 months in patients with relapsed and refractory Multiple Myeloma. Based on this background the authors conducted this randomized, open label, multicenter, phase III study in which the safety and efficacy of a combination of KYPROLIS® (Carfilzomib), REVLIMID® and weekly Dexamethasone (KYPROLIS® group) was compared with a combination of REVLIMID® and weekly Dexamethasone (control group), in patients with relapsed Multiple Myeloma. Seven hundred and ninety two (N=792) patients were randomly assigned in a 1:1 ratio to KYPROLIS® group (N=396) and control group (N=396). Eligible patients included those with Multiple Myeloma who had received one to three prior treatments which included VELCADE® or REVLIMID and Dexamethasone combination, provided that they did not have disease progression during treatment with these agents. The 28 day treatment cycle consisted of KYPROLIS® IV given on days 1, 2, 8, 9, 15, and 16 (starting dose, 20 mg/m2 on days 1 and 2 of cycle 1 with a target dose of 27 mg/m2 thereafter) during cycles 1 through 12 and on days 1, 2, 15, and 16 during cycles 13 through 18, following which KYPROLIS® was discontinued. REVLIMID® 25 mg PO was given on days 1 through 21 and Dexamethasone 40 mg PO was administered on days 1, 8, 15, and 22. Patients in both treatment groups received only REVLIMID® and Dexamethasone after cycle 18 until disease progression. Antiviral and antithrombotic prophylaxis was administered to patients in both treatment groups. The primary end point was Progression Free Survival and secondary end points included Overall Survival, the rate of overall response (partial response or better), response duration, health-related quality of life, and safety. The rate of clinical benefit (minimal response or better) was an exploratory end point. The study met its primary endpoint at the time of the pre-specified interim analysis with a significant improvement in the median Progression Free Survival for those patients in the KYPROLIS® group compared to the control group (26.3 months versus 17.6 months; HR=0.69; P=0.0001). This benefit in the PFS was demonstrated across all predefined subgroups. The median overall survival was not reached in either group and the 24 month overall survival rates were 73.3% and 65.0% in the KYPROLIS® and control groups, respectively (HR=0.79; P=0.04). The overall response rates (partial response or better) were 87.1% and 66.7% in the KYPROLIS® and control groups, respectively (P<0.001). Amongst the responders, 31.8% and 9.3% of patients in the respective groups had a complete response or better and 14.1% and 4.3% had a stringent complete response. Further, patients in the KYPROLIS® group reported superior health-related quality of life. Grade 3 or higher adverse events were reported in 83.7% and 80.7% of patients in the KYPROLIS® and control groups respectively. The authors concluded that the addition of KYPROLIS® to REVLIMID® and Dexamethasone resulted in significant improvement in PFS as compared with REVLIMID® and Dexamethasone alone, in patients with relapsed Multiple Myeloma. Additional benefits in the KYPROLIS® group included higher and deep response rates, improved health-related quality of life, a favorable risk–benefit profile and a trend towards improved Overall Survival. Stewart AK, Rajkumar SV, Dimopoulos MA, et al. N Engl J Med 2015; 372:142-152
Category: Hem/Onc Updates
Adjuvant Paclitaxel and Trastuzumab for Node-Negative, HER2-Positive Breast Cancer
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their life time. Approximately, 233,000 new cases of invasive breast cancer were diagnosed in 2014 and 40,000 women died of the disease. The HER or erbB family of receptors consist of HER1, HER2, HER3 and HER4. Approximately 15%-20% of invasive breast cancers overexpress HER2/neu oncogene, which is a negative predictor of outcomes without systemic therapy. HERCEPTIN® (Trastuzumab) is a humanized monoclonal antibody targeting HER2.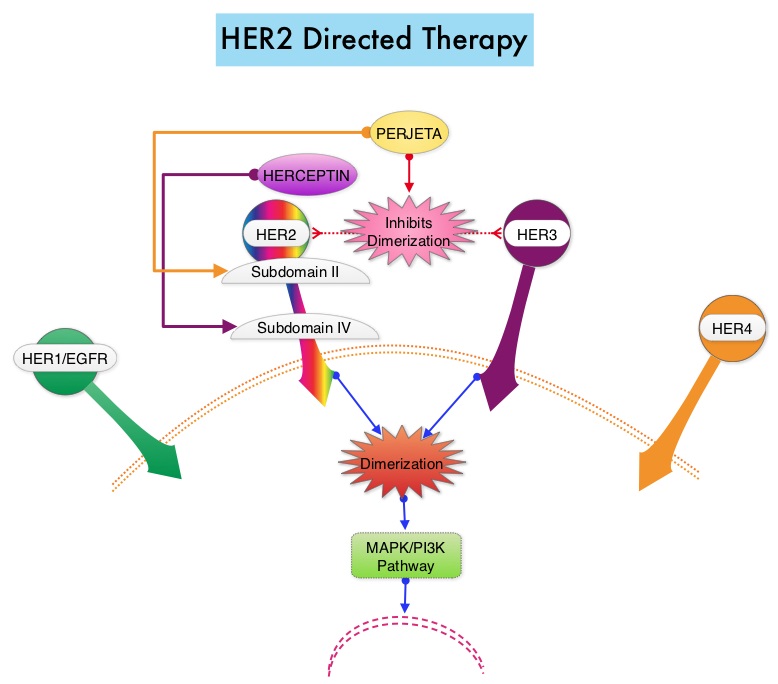 HERCEPTIN® binds to subdomain IV of the HER2 extracellular domain and blocks the downstream cell signaling pathways (PI3K-AKT pathway) and induces Antibody Dependent Cellular Cytotoxicity (ADCC). HERCEPTIN® in combination with chemotherapy has been proven to significantly improve Progression Free Survival and Overall Survival in patients with advanced breast cancer. Adjuvant chemotherapy in combination with HERCEPTIN® has been shown to reduce the relative risk of relapse by 52% and relative risk of death by 33%. The National Comprehensive Cancer Network (NCCN) has recommended adjuvant chemotherapy with HERCEPTIN® for patients with small, HER positive, node-negative tumors, including those with T1bN0 tumors, even though there are little or no data supporting this recommendation, because these patients are generally not included in adjuvant therapy studies. Further, the chemotherapy regimens often recommended (ACTH, TCH) along with HERCEPTIN® are relatively toxic. The authors in this study chose a less toxic chemotherapy regimen than the regimens often recommended for those patients with high risk disease. In this multicenter, investigator initiated study, 406 patients with tumors measuring up to 3 cm in greatest dimension received weekly treatment with TAXOL® (Paclitaxel) and HERCEPTIN (Trastuzumab) for 12 weeks, followed by 9 months of HERCEPTIN® monotherapy. Close to 50% of the patients had tumors 1 cm in diameter or less, about 40% of the patients had tumors 1-2 cm in diameter and majority of the tumors (56%) were high grade. Treatment regimen consisted of TAXOL® 80 mg/m2 IV weekly, for 12 weeks and HERCEPTIN® 4 mg/kg loading dose IV on day 1, followed by 2 mg/kg weekly, for a total of 12 doses followed by HERCEPTIN® 6 mg/kg every 3 weeks for an additional 40 weeks, for a total of 52 weeks of treatment with HERCEPTIN®. Patients who underwent lumpectomy received either partial breast radiation before the initiation of the therapy, or radiation of the whole breast, following completion of treatment with TAXOL®. Treatment with HERCEPTIN® was continued during the time patient was receiving radiation therapy. Adjuvant hormonal therapy was recommended for women with hormone-receptor positive tumors after the completion of TAXOL® treatment. The primary end point was survival free from invasive disease. The median follow up period was 4 years. The 3-year rate of survival free from invasive disease was 98.7%. Treatment was very well tolerated with a low incidence of heart failure (0.5%) and neuropathy. The authors concluded that a less toxic regimen such as HERCEPTIN® given along with weekly TAXOL® has significant efficacy, decreasing the risk of recurrence in this patient group, most notable during the first three years after diagnosis. They also point out that the risk of recurrence of breast cancer is greatest during the first 3-5 years after diagnosis and it would seem unlikely that a different chemotherapy regimen administered with HERCEPTIN® would impact the risk of late recurrences. Tolaney SM, Barry WT, Dang CT, et al. N Engl J Med 2015;372:134-141
HERCEPTIN® binds to subdomain IV of the HER2 extracellular domain and blocks the downstream cell signaling pathways (PI3K-AKT pathway) and induces Antibody Dependent Cellular Cytotoxicity (ADCC). HERCEPTIN® in combination with chemotherapy has been proven to significantly improve Progression Free Survival and Overall Survival in patients with advanced breast cancer. Adjuvant chemotherapy in combination with HERCEPTIN® has been shown to reduce the relative risk of relapse by 52% and relative risk of death by 33%. The National Comprehensive Cancer Network (NCCN) has recommended adjuvant chemotherapy with HERCEPTIN® for patients with small, HER positive, node-negative tumors, including those with T1bN0 tumors, even though there are little or no data supporting this recommendation, because these patients are generally not included in adjuvant therapy studies. Further, the chemotherapy regimens often recommended (ACTH, TCH) along with HERCEPTIN® are relatively toxic. The authors in this study chose a less toxic chemotherapy regimen than the regimens often recommended for those patients with high risk disease. In this multicenter, investigator initiated study, 406 patients with tumors measuring up to 3 cm in greatest dimension received weekly treatment with TAXOL® (Paclitaxel) and HERCEPTIN (Trastuzumab) for 12 weeks, followed by 9 months of HERCEPTIN® monotherapy. Close to 50% of the patients had tumors 1 cm in diameter or less, about 40% of the patients had tumors 1-2 cm in diameter and majority of the tumors (56%) were high grade. Treatment regimen consisted of TAXOL® 80 mg/m2 IV weekly, for 12 weeks and HERCEPTIN® 4 mg/kg loading dose IV on day 1, followed by 2 mg/kg weekly, for a total of 12 doses followed by HERCEPTIN® 6 mg/kg every 3 weeks for an additional 40 weeks, for a total of 52 weeks of treatment with HERCEPTIN®. Patients who underwent lumpectomy received either partial breast radiation before the initiation of the therapy, or radiation of the whole breast, following completion of treatment with TAXOL®. Treatment with HERCEPTIN® was continued during the time patient was receiving radiation therapy. Adjuvant hormonal therapy was recommended for women with hormone-receptor positive tumors after the completion of TAXOL® treatment. The primary end point was survival free from invasive disease. The median follow up period was 4 years. The 3-year rate of survival free from invasive disease was 98.7%. Treatment was very well tolerated with a low incidence of heart failure (0.5%) and neuropathy. The authors concluded that a less toxic regimen such as HERCEPTIN® given along with weekly TAXOL® has significant efficacy, decreasing the risk of recurrence in this patient group, most notable during the first three years after diagnosis. They also point out that the risk of recurrence of breast cancer is greatest during the first 3-5 years after diagnosis and it would seem unlikely that a different chemotherapy regimen administered with HERCEPTIN® would impact the risk of late recurrences. Tolaney SM, Barry WT, Dang CT, et al. N Engl J Med 2015;372:134-141
Hereditary Hemochromatosis Missed Diagnosis or Misdiagnosis?
SUMMARY: It is estimated that 1-6 individuals per 100 in the United States have Iron overload syndrome as measured by random elevated Iron Saturation level. Hereditary Hemochromatosis (HH) is an inherited, Autosomal Recessive, iron storage disease and in the US usually occurs as a result of HFE gene mutations. This is more prevalent among persons of European origin and the HFE gene is located on the short arm of chromosome 6 and modulates iron uptake. Two mutations on the HFE gene, C282Y and H63D, account for the majority of the cases of Hereditary Hemochromatosis, in the United States.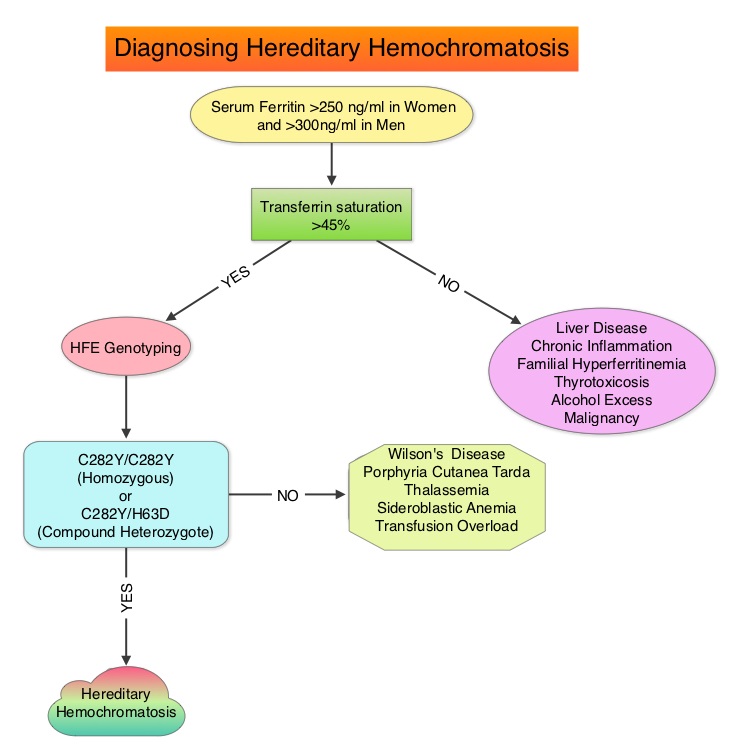 The authors in this provocative study reviewed the electronic medical records of patients seen at a tertiary referral center, to evaluate the accuracy of diagnosis of Hereditary Hemochromatosis (HH). HFE genotyping helps differentiate HH from secondary causes of Iron overload syndromes. This differentiation is relevant because of the lack of established guidelines with regards to management of individuals with abnormal iron studies, secondary to liver disease. It is however well known that HH and iron overload related to transfusions, can cause organ damage and this could be prevented by removing the excess iron. In this study, the authors investigated the diagnostic approach, when elevated iron studies were noted, interpretation of HFE genotyping results by physicians in order to accurately diagnose HH and factors contributing to misdiagnosis. Their review conducted between January 2002 and May 2012, demonstrated that of the 601 patients with disorders of iron metabolism, only 62% were genotyped for mutations in the HFE gene. Of those genotyped, 54% had genotypes consistent with HH (Homozygotes – C282Y/C282Y or Compound Heterozygotes – C282Y/H63D) and the rest of the 46% had non-hereditary hemochromatosis genotypes (C282Y heterozygotes, H63D homozygotes and heterozygotes, etc). One half of these non-hereditary hemochromatosis genotype patients were misdiagnosed as Hereditary Hemochromatosis and a third of these patients underwent phlebotomy. Of those who were not genotyped for mutations in the HFE gene, a third of these patients were diagnosed to have Hereditary Hemochromatosis and majority of these patients underwent phlebotomy. More than two third of the patients misdiagnosed to have Hereditary Hemochromatosis in fact had liver disease and 5% had other hematological conditions. The authors concluded that C282Y heterozygotes as well as H63D homozygotes and heterozygotes do not have Hereditary Hemochromatosis and a majority of patients with iron overload are misdiagnosed to have Hereditary Hemochromatosis. Aggressive phlebotomy in the absence of an appropriate Hereditary Hemochromatosis genotype, is not indicated and can be potentially harmful, delaying more appropriate therapy. Cherfane CE, Hollenbeck RD, Go J, et al. The American Journal of Medicine 2013;126:1010-1015.
The authors in this provocative study reviewed the electronic medical records of patients seen at a tertiary referral center, to evaluate the accuracy of diagnosis of Hereditary Hemochromatosis (HH). HFE genotyping helps differentiate HH from secondary causes of Iron overload syndromes. This differentiation is relevant because of the lack of established guidelines with regards to management of individuals with abnormal iron studies, secondary to liver disease. It is however well known that HH and iron overload related to transfusions, can cause organ damage and this could be prevented by removing the excess iron. In this study, the authors investigated the diagnostic approach, when elevated iron studies were noted, interpretation of HFE genotyping results by physicians in order to accurately diagnose HH and factors contributing to misdiagnosis. Their review conducted between January 2002 and May 2012, demonstrated that of the 601 patients with disorders of iron metabolism, only 62% were genotyped for mutations in the HFE gene. Of those genotyped, 54% had genotypes consistent with HH (Homozygotes – C282Y/C282Y or Compound Heterozygotes – C282Y/H63D) and the rest of the 46% had non-hereditary hemochromatosis genotypes (C282Y heterozygotes, H63D homozygotes and heterozygotes, etc). One half of these non-hereditary hemochromatosis genotype patients were misdiagnosed as Hereditary Hemochromatosis and a third of these patients underwent phlebotomy. Of those who were not genotyped for mutations in the HFE gene, a third of these patients were diagnosed to have Hereditary Hemochromatosis and majority of these patients underwent phlebotomy. More than two third of the patients misdiagnosed to have Hereditary Hemochromatosis in fact had liver disease and 5% had other hematological conditions. The authors concluded that C282Y heterozygotes as well as H63D homozygotes and heterozygotes do not have Hereditary Hemochromatosis and a majority of patients with iron overload are misdiagnosed to have Hereditary Hemochromatosis. Aggressive phlebotomy in the absence of an appropriate Hereditary Hemochromatosis genotype, is not indicated and can be potentially harmful, delaying more appropriate therapy. Cherfane CE, Hollenbeck RD, Go J, et al. The American Journal of Medicine 2013;126:1010-1015.
Adjuvant and Salvage Radiotherapy after Prostatectomy American Society of Clinical Oncology Clinical Practice Guideline Endorsement
SUMMARY: The American Society of Clinical Oncology (ASCO) recently endorsed the Clinical Practice Guidelines recommended by the American Urological Association (AUA)/American Society for Radiation Oncology (ASTRO), on Adjuvant and Salvage Radiotherapy after Prostatectomy. These guidelines target Medical and Radiation Oncologists, Primary care providers, Urologists, other health care providers and address patient counseling, use of radiotherapy in the adjuvant and salvage settings, definition of biochemical recurrence and restaging evaluation. The following are the ASCO Key Recommendations for Adjuvant and Salvage Radiotherapy after Prostatectomy:
1. Patients who are being considered for management of localized prostate cancer with radical prostatectomy should be informed of the potential for adverse pathologic findings that portend a higher risk of cancer recurrence and that these findings may suggest a potential benefit of additional therapy after surgery.
2. Patients with adverse pathologic findings, including seminal vesicle invasion, positive surgical margins, and extraprostatic extension, should be informed that adjuvant radiotherapy, compared with radical prostatectomy only, reduces the risk of biochemical Prostate Specific Antigen (PSA) recurrence, local recurrence, and clinical progression of cancer. They should also be informed that the impact of adjuvant radiotherapy on subsequent metastases and overall survival is less clear; one of two randomized controlled trials that addressed these outcomes indicated a benefit, but the other trial did not demonstrate a benefit defined as reduced risk of metastasis and death.
3. Physicians should “OFFER” adjuvant radiotherapy to patients with adverse pathologic findings at prostatectomy, including seminal vesicle invasion, positive surgical margins, or extraprostatic extension, because of demonstrated reductions in biochemical recurrence, local recurrence and clinical progression.
4. Patients should be informed that the development of a PSA recurrence after surgery is associated with a higher risk of development of metastatic prostate cancer or death resulting from the disease. Congruent with this clinical principle, physicians should regularly monitor PSA after radical prostatectomy to enable early administration of salvage therapies if appropriate.
5. Clinicians should define biochemical recurrence as a detectable or increasing PSA value after surgery that is more than 0.2 ng/mL, with a second confirmatory level more than 0.2 ng/mL.
6. A restaging evaluation in a patient with a PSA recurrence may be considered although it is not clear at this time which imaging modalities to use, as all imaging modalities have limited sensitivity and specificity in the low PSA range.
7. Physicians should “OFFER” salvage radiotherapy to patients with PSA or local recurrence after radical prostatectomy, in whom there is no evidence of distant metastatic disease.
8. Patients should be informed that the effectiveness of radiotherapy for PSA recurrence is greatest when administered at lower levels of PSA (less than 1 ng/ml). Salvage radiotherapy in this patient population with a short PSA doubling time, has been shown to improve overall survival.
9. Patients should be informed of the possible short and long term urinary, bowel, and sexual adverse effects of radiotherapy as well as of the potential benefits of controlling disease recurrence.
This endorsement was made with certain qualifying statements, clarifying certain aspects of these guidelines.
a) The word “OFFER” should be interpreted as having a detailed discussion with the patient about the risks and benefits of adjuvant radiation.
b) Even though 0.2 ng/mL is considered a reasonable cut point for PSA recurrence, the benefits of using this cut point versus other cut points remains unclear.
c) Patient’s who have the greatest benefit in absolute risk reduction from adjuvant RT, are those with adverse pathologic findings as noted in the guidelines, with a high risk of recurrence or clinical progression.
In conclusion, the decision to administer adjuvant or salvage radiotherapy should be made by the patient and multidisciplinary treatment team, after discussing the risks and benefits of such intervention. Freedland SJ, Rumble RB, Finelli A, et al. J Clin Oncol 2014;32:3892-3898
The Second ASH CHOOSING WISELY® Campaign Five Hematologic Tests and Treatments to Question
SUMMARY: CHOOSING WISELY® is a quality improvement initiative led by the American Board of Internal Medicine Foundation in collaboration with leading medical societies in the United States such as the American Society of Hematology (ASH). This organization was established to improve quality of medical care, after it was noted that about 25% of the tests ordered at the time of hospital admission and 65% of the tests ordered on subsequent days were avoidable. Further, there is ample evidence to suggest that reducing unneeded investigations can decrease costs, increase patient satisfaction and quality of care. CHOOSING WISELY® has challenged medical societies to identify 5 tests, procedures or treatments, within each specialty's clinical domain, that are offered to patients, despite the lack of evidence demonstrating its benefit. The goal is to make positive changes in the actual delivery of patient care. The 2014 Task Force was comprised of 13 individuals representing a broad spectrum of hematologic expertise including malignant, benign, adult, and pediatric specialists. The five final recommendations of the 2014 ASH Choosing Wisely Campaign are summarized below. Practicing hematologists should give due consideration to these recommendations which are evidence based and cost effective.
ASH recommendation #1: In patients with a first VTE (Venous ThromboEmbolism) provoked by a major, transient VTE risk factor such as surgery, trauma, or an intravascular catheter, do not treat with an anticoagulant for more than 3 months. There is a low risk of VTE recurrence after three months in this setting and anticoagulation for VTE continued beyond three months may be associated with increased bleeding risk, particularly in the elderly and those with comorbidities. This recommendation is not applicable to patients with non-major, transient VTE risk factors such as travel-associated immobility, pregnancy or hormone use. Women who experience a first VTE during pregnancy should receive anticoagulation until at least six weeks post-partum, for a minimum total duration of three months or longer. VTEs occurring in the context of estrogen supplements are associated with a low recurrence rate following discontinuation of hormonal therapy/oral contraceptives and three months of anticoagulation may be adequate. However, the optimal duration of anticoagulation for VTEs provoked by hormones or by travel remains unclear and should be determined on a on a case-by-case basis.
ASH recommendation #2: Routine transfusion of PRBC for chronic anemia or uncomplicated pain crises in patients with sickle cell disease is not recommended as these patients who are predominantly African Americans, are at an especially higher risk for alloimmunization to minor blood group antigens, which can result in delayed-hemolytic transfusion reactions, as well as difficulty finding compatible blood when necessary. The baseline hemoglobin values range between 7 and 10g/dL in stable patients with severe sickle cell disease and these patients are often able to tolerate a 1-2g/dL decreases in their hemoglobin values following IV hydration. Further, data does not strongly support that episodic red cell transfusion reduces pain during acute vaso-occlusive crises. Moreover, iron overload from repeated transfusions can cause significant morbidity and mortality in patients with sickle cell disease.
ASH recommendation #3: Unlike in other lymphoproliferative diseases, routine surveillance CT scans are not recommended in patients with asymptomatic, early stage chronic lymphocytic leukemia (CLL). Both the Rai and Binet staging systems are based on physical exam findings and complete blood counts and prognosis can be assessed with molecular mutational analyses. CT scans are therefore not necessary and can be potentially harmful, by exposing patients to radiation and may also trigger additional workup to evaluate incidental findings (Cascade effect), that may not be of importance.
ASH recommendation #4: Do not test or treat for suspected Heparin-Induced Thrombocytopenia (HIT) in patients with a Low pretest probability of HIT. The 4Ts is a pretest scoring system for HIT and incorporates 4 components of HIT which include magnitude of thrombocytopenia, timing of thrombocytopenia with respect to heparin exposure, thrombosis or other sequelae of HIT and likelihood of other causes of thrombocytopenia. The 4Ts score is the sum of the values for each of the 4 categories. A score of 0-3 is classified as Low, 4-5 as Intermediate and 6-8 as High pretest probability for HIT. The negative predictive value of a Low 4T’s score is close to 100% in adults. Further, Enzyme ImmunoAssays (EIA) for HIT have a high false positive rate and a positive EIA HIT test results in a patient with a Low 4T’s score is much more likely to represent a false positive value than true positive. Confirmatory testing with serotonin release assays are not easily available and can be expensive. Misdiagnosing HIT can harm patients by denying them a heparin preparation in the future and the use of alternative, expensive anticoagulants such as Argatroban in these thrombocytopenic patients can be associated with a higher risk of bleeding. For these reasons, testing for HIT is only cost-effective when the pre-test probability of HIT is greater than 8%, which corresponds to an Intermediate or High 4T’s score
ASH recommendation #5: Do not treat patients with Immune Thrombocytopenic Purpura (ITP) in the absence of bleeding or a very low platelet count. ITP is often a temporary condition in children and resolves without treatment and treatment is not recommended in childhood ITP unless there is bleeding or risk factors for bleeding. ITP in adults is usually a chronic disease with remissions and exacerbations and patients with a platelet count of 30,000/microL or more and with no bleeding, can be observed without intervention. Steroids can impair glucose metabolism, increase infection risk, cause adrenal suppression and in children can cause growth impairment. Splenectomy is associated with perioperative risks and small risk of life threatening infections. Rituximab can cause Hepatitis B reactivation and TPO receptor agonists are only cost-effective in the setting of severe ITP, refractory to other treatment interventions.
Hicks LK, Bering H, Carson KR, et al. Prepublished online December 3, 2014; doi:10.1182/blood-2014-09-599399
A phase 3 randomized, open-label study of nivolumab (anti-PD-1;BMS-936558; ONO-4538) versus investigator's choice chemotherapy (ICC) in patients with advanced melanoma after prior anti-CTLA-4 therapy
SUMMARY: The FDA on December 22, 2014 granted accelerated approval to OPDIVO® (Nivolumab) for the treatment of patients with unresectable or metastatic melanoma whose disease has progressed following YERVOY® (Ipilimumab) and if BRAF V600 mutation positive, a BRAF inhibitor. It is estimated that in the US, approximately 76,000 new cases of melanoma will be diagnosed and close to 8000 individuals will die of the disease in 2014. The incidence of melanoma has been on the rise for the past three decades. Unlike other malignancies, the role of chemotherapy for the treatment of melanoma has been limited. Treatment of advanced melanoma with immunotherapy using a cytokine, Interleukin-2 (IL-2) produced by T cells during an immune response, was first explored in the mid 1970’s. Durable responses were noted in a very small percentage of patients but this was associated with significant toxicities. This however opened the doors for the development a novel immunotherapeutic approaches, with a better understanding of the Immune checkpoints. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent, related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4), also known as CD152, PD-1(Programmed cell Death-1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. The first immune checkpoint protein to be clinically targeted was CTLA-4. YERVOY® (Ipilimumab), an antibody that blocks Immune checkpoint protein/receptor CTLA- 4, has been shown to prolong overall survival in patients with previously treated, unresectable or metastatic melanoma. OPDIVO® (Nivolumab) is a fully human, immunoglobulin G4 monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the T cells. OPDIVO® in previously conducted studies demonstrated durable antitumor activity and promising overall survival (OS) in pretreated patients. CheckMate-037 is an open label, randomized, phase III study, in which 370 patients with unresectable or metastatic melanoma, received OPDIVO® 3 mg/kg IV every 2 weeks (N=268) or investigator’s choice of chemotherapy, which included either Dacarbazine or a combination of Carboplatin plus Paclitaxel given every 3 weeks (N=102). Treatment was continued until disease progression or unacceptable toxicity. Eligible patients were required to have disease progression following YERVOY® (Ipilimumab) and a BRAF inhibitor if BRAF V600 mutation positive. The primary endpoints were ORR and overall survival. Early findings (Objective Response Rate-ORR) in the first 120 patients who were treated with OPDIVO® and in 47 patients treated with chemotherapy and had a minimum 6 months follow up (planned interim analysis), was presented at the 2014 ESMO Congress. The Objective Response Rate (ORR) was 32% in the OPDIVO® group and 11% in the chemotherapy group. The median time to response was 2.1 months in the OPDIVO® group and 3.5 months with chemotherapy. The majority, (95%) of responses at 6 months were ongoing in the OPDIVO® group and the median duration of response was not reached. The most common (greater than or equal to 20%) adverse reaction in the OPDIVO® group was rash. Grade 3 and 4 adverse events were seen in 2-5% of patients receiving OPDIVO® and included abdominal pain, hyponatremia, elevated liver enzymes and increased lipase. Clinically significant immune-mediated adverse reactions were pneumonitis, colitis, hepatitis, nephritis, and thyroid dysfunction. OPDIVO® is a new and novel treatment option for patients with advanced melanoma and is a welcome addition, as we try to better understand tumor immunology. Weber JS, Minor DR, D'Angelo S, et al. ESMO 2014, LBA3_PR
The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4), also known as CD152, PD-1(Programmed cell Death-1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. The first immune checkpoint protein to be clinically targeted was CTLA-4. YERVOY® (Ipilimumab), an antibody that blocks Immune checkpoint protein/receptor CTLA- 4, has been shown to prolong overall survival in patients with previously treated, unresectable or metastatic melanoma. OPDIVO® (Nivolumab) is a fully human, immunoglobulin G4 monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the T cells. OPDIVO® in previously conducted studies demonstrated durable antitumor activity and promising overall survival (OS) in pretreated patients. CheckMate-037 is an open label, randomized, phase III study, in which 370 patients with unresectable or metastatic melanoma, received OPDIVO® 3 mg/kg IV every 2 weeks (N=268) or investigator’s choice of chemotherapy, which included either Dacarbazine or a combination of Carboplatin plus Paclitaxel given every 3 weeks (N=102). Treatment was continued until disease progression or unacceptable toxicity. Eligible patients were required to have disease progression following YERVOY® (Ipilimumab) and a BRAF inhibitor if BRAF V600 mutation positive. The primary endpoints were ORR and overall survival. Early findings (Objective Response Rate-ORR) in the first 120 patients who were treated with OPDIVO® and in 47 patients treated with chemotherapy and had a minimum 6 months follow up (planned interim analysis), was presented at the 2014 ESMO Congress. The Objective Response Rate (ORR) was 32% in the OPDIVO® group and 11% in the chemotherapy group. The median time to response was 2.1 months in the OPDIVO® group and 3.5 months with chemotherapy. The majority, (95%) of responses at 6 months were ongoing in the OPDIVO® group and the median duration of response was not reached. The most common (greater than or equal to 20%) adverse reaction in the OPDIVO® group was rash. Grade 3 and 4 adverse events were seen in 2-5% of patients receiving OPDIVO® and included abdominal pain, hyponatremia, elevated liver enzymes and increased lipase. Clinically significant immune-mediated adverse reactions were pneumonitis, colitis, hepatitis, nephritis, and thyroid dysfunction. OPDIVO® is a new and novel treatment option for patients with advanced melanoma and is a welcome addition, as we try to better understand tumor immunology. Weber JS, Minor DR, D'Angelo S, et al. ESMO 2014, LBA3_PR
Prognostic significance of FLT3 internal tandem duplication, nucleophosmin 1, and CEBPA gene mutations for acute myeloid leukemia patients with normal karyotype and younger than 60 years a systematic review and meta-analysis
SUMMARY: The American Cancer Society estimates that in 2014, 18,860 new cases of Acute Myeloid Leukemia (AML) will be diagnosed in the United States and 10,460 patients will die of the disease. Acute Myeloid Leukemia in general is a disease of the elderly and the average age of a patient with AML is about 66 years. AML can be considered as a group of heterogeneous diseases with different clinical behavior and outcomes. Cytogenetic analysis has been part of routine evaluation when caring for patients with AML. By predicting resistance to therapy, tumor cytogenetics will stratify patients, based on risk and help manage them accordingly. Even though cytotoxic chemotherapy may lead to long term remission and cure in a minority of patients with favorable cytogenetics, patients with high risk features such as unfavorable cytogenetics, molecular abnormalities, prior myelodysplasia and advanced age, have poor outcomes with conventional chemotherapy.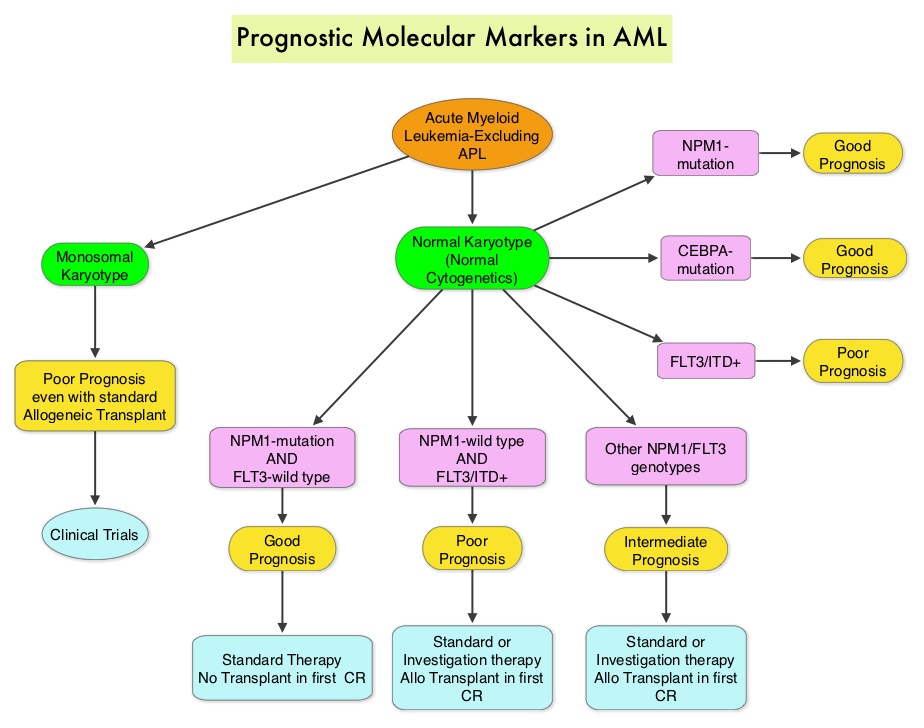 The Fms-Like Tyrosine kinase 3 (FLT3) is a receptor tyrosine kinase in the PDGF family of growth factor receptors located on the cell surface (transmembrane) and plays an important role in both normal and malignant hematopoiesis by activating key signaling pathways. Activating mutations in the FLT3 receptor is the most common genetic abnormality in AML and is detected in approximately 30% of the patients. The most common FLT3 mutation is the FLT3-ITD (Internal Tandem Duplication) mutation caused by tandem duplication within the coding region of the gene. The presence of FLT3-ITD mutations can negate the benefit of any other favorable molecular and cytogenetic features. Patients with FLT3-ITD mutations are predicted to have poor outcomes with shorter remission duration and significantly decreased leukemia free and overall survival. These mutations are detected using Polymerase Chain Reaction (PCR) based molecular diagnostic DNA testing. The authors in this meta-analysis examined the prognostic significance of three mutations frequently noted in patients with cytogenetically normal Acute Myeloid Leukemia. These mutations included FLT3-ITD, mutated NPM1 (Nucleophosmin) and mutations of the CCAAT enhancer-binding protein alpha (CEBPA) gene. This systematic review and meta-analysis included 1942 patients from multiple electronic databases from 2000 to March 2012. It was noted that FLT3-ITD was associated with the worse prognosis, with inferior Overall Survival (OS) and Relapse Free Survival (RFS), whereas mutations in NPM1 and CEBPA genes were associated with a favorable prognosis. The discovery of new molecular mutations in AML patients with normal cytogenetics may help predict outcomes and provide valuable information to facilitate risk-adapted therapy. Port M, Böttcher M, Thol F, et al. Ann Hematol. 2014;93:1279-1286
The Fms-Like Tyrosine kinase 3 (FLT3) is a receptor tyrosine kinase in the PDGF family of growth factor receptors located on the cell surface (transmembrane) and plays an important role in both normal and malignant hematopoiesis by activating key signaling pathways. Activating mutations in the FLT3 receptor is the most common genetic abnormality in AML and is detected in approximately 30% of the patients. The most common FLT3 mutation is the FLT3-ITD (Internal Tandem Duplication) mutation caused by tandem duplication within the coding region of the gene. The presence of FLT3-ITD mutations can negate the benefit of any other favorable molecular and cytogenetic features. Patients with FLT3-ITD mutations are predicted to have poor outcomes with shorter remission duration and significantly decreased leukemia free and overall survival. These mutations are detected using Polymerase Chain Reaction (PCR) based molecular diagnostic DNA testing. The authors in this meta-analysis examined the prognostic significance of three mutations frequently noted in patients with cytogenetically normal Acute Myeloid Leukemia. These mutations included FLT3-ITD, mutated NPM1 (Nucleophosmin) and mutations of the CCAAT enhancer-binding protein alpha (CEBPA) gene. This systematic review and meta-analysis included 1942 patients from multiple electronic databases from 2000 to March 2012. It was noted that FLT3-ITD was associated with the worse prognosis, with inferior Overall Survival (OS) and Relapse Free Survival (RFS), whereas mutations in NPM1 and CEBPA genes were associated with a favorable prognosis. The discovery of new molecular mutations in AML patients with normal cytogenetics may help predict outcomes and provide valuable information to facilitate risk-adapted therapy. Port M, Böttcher M, Thol F, et al. Ann Hematol. 2014;93:1279-1286
Olaparib Monotherapy in Patients with Advanced Cancer and a Germline BRCA1/2 Mutation
SUMMARY:The FDA on December 19, 2014 approved LYNPARZA® (Olaparib) as monotherapy for the treatment of patients with deleterious or suspected deleterious germline BRCA mutated (gBRCAm) advanced ovarian cancer who had been treated with three or more prior lines of chemotherapy. It is estimated that in the United States, approximately 22,000 women will be diagnosed with ovarian cancer in 2014 and a little over 14,000 women will die of the disease. DNA can be damaged due to errors during its replication or as a result of environmental exposure to ultraviolet radiation from the sun or other toxins. The tumor suppressor genes such as BRCA1 (Breast Cancer 1) and BRCA2 help repair damaged DNA and thus play an important role in maintaining cellular genetic integrity, failing which these genetic aberrations can result in malignancies. The BRCA1 gene is located on the long (q) arm of chromosome 17 whereas BRCA2 is located on the long arm of chromosome 13. Mutations in BRCA1 and BRCA2 account for about 20 to 25 percent of hereditary breast cancers and about 5 to 10 percent of all breast cancers. They also account 15 percent of ovarian cancers in addition to other cancers such as colon and prostate. These mutations can be inherited from either of the parents and a child has a 50 percent chance of inheriting this mutation and the deleterious effects of the mutations are seen even when an individual’s second copy of the gene is normal.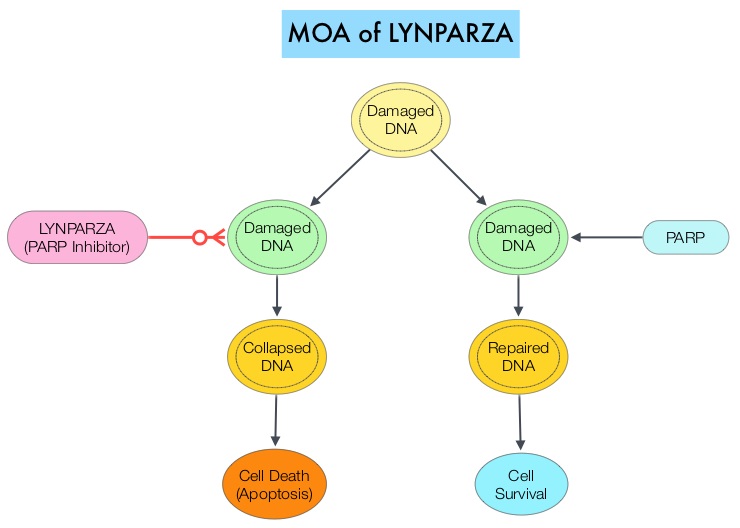 The PARP (Poly ADP Ribose Polymerase) family of enzymes which include PARP1 and PARP2, repair damaged DNA. LYNPARZA® is a PARP enzyme inhibitor that causes cell death in tumors that already have a DNA repair defect, such as those with BRCA1 and BRCA2 mutations. The approval of LYNPARZA® was based on a single arm phase II trial in which 137 platinum resistant ovarian cancer patients with measurable germline BRCA mutations were enrolled. The BRCA mutation status was verified retrospectively in 97% of the patients with available blood samples from the phase II study, using the BRACAnalysis CDx® test. These patients had received three or more lines of prior chemotherapy. Treatment consisted of LYNPARZA® administered orally twice a day and was continued until disease progression or unacceptable toxicity. The primary endpoint was Objective Response Rate (ORR). The Overall Response Rate was 34% and the median response duration was 7.9 months. In a larger cohort of patients reported by the authors (ovarian cancer cohort, N=193) the median Progression Free Survival was 7 months, 55% of patients were progression free at 6 months, the median Overall Survival was 16.6 months and 64.4% of patients were alive at 12 months. The most common adverse reactions associated with LYNPARZA® were anemia, nausea, fatigue (including asthenia), vomiting, diarrhea, dysgeusia, dyspepsia, headache, decreased appetite, nasopharyngitis/pharyngitis/URI, cough, arthralgia/musculoskeletal pain, myalgia, back pain, dermatitis/rash and abdominal pain/discomfort. This ground breaking therapy with LYNPARZA® is first of a new class of drugs, for treating ovarian cancer and along with the BRACAnalysis CDx® companion diagnostic test, is a significant milestone for patients with difficult-to-treat advanced ovarian cancer, with germline BRCA mutations. Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. [published online November 3, 2014]. J Clin Oncol. doi:10.1200/JCO.2014.56.2728.
The PARP (Poly ADP Ribose Polymerase) family of enzymes which include PARP1 and PARP2, repair damaged DNA. LYNPARZA® is a PARP enzyme inhibitor that causes cell death in tumors that already have a DNA repair defect, such as those with BRCA1 and BRCA2 mutations. The approval of LYNPARZA® was based on a single arm phase II trial in which 137 platinum resistant ovarian cancer patients with measurable germline BRCA mutations were enrolled. The BRCA mutation status was verified retrospectively in 97% of the patients with available blood samples from the phase II study, using the BRACAnalysis CDx® test. These patients had received three or more lines of prior chemotherapy. Treatment consisted of LYNPARZA® administered orally twice a day and was continued until disease progression or unacceptable toxicity. The primary endpoint was Objective Response Rate (ORR). The Overall Response Rate was 34% and the median response duration was 7.9 months. In a larger cohort of patients reported by the authors (ovarian cancer cohort, N=193) the median Progression Free Survival was 7 months, 55% of patients were progression free at 6 months, the median Overall Survival was 16.6 months and 64.4% of patients were alive at 12 months. The most common adverse reactions associated with LYNPARZA® were anemia, nausea, fatigue (including asthenia), vomiting, diarrhea, dysgeusia, dyspepsia, headache, decreased appetite, nasopharyngitis/pharyngitis/URI, cough, arthralgia/musculoskeletal pain, myalgia, back pain, dermatitis/rash and abdominal pain/discomfort. This ground breaking therapy with LYNPARZA® is first of a new class of drugs, for treating ovarian cancer and along with the BRACAnalysis CDx® companion diagnostic test, is a significant milestone for patients with difficult-to-treat advanced ovarian cancer, with germline BRCA mutations. Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. [published online November 3, 2014]. J Clin Oncol. doi:10.1200/JCO.2014.56.2728.
Impact on overall survival (OS) with chemohormonal therapy versus hormonal therapy for hormone-sensitive newly metastatic prostate cancer (mPrCa) An ECOG-led phase III randomized trial
SUMMARY: Prostate cancer is the most common cancer in American men, excluding skin cancer and 1 in 7 men will be diagnosed with prostate cancer during their lifetime. It is estimated that in the United States, over 230,000 new cases of prostate cancer will be diagnosed in 2014 and close to 30,000 men will die of the disease.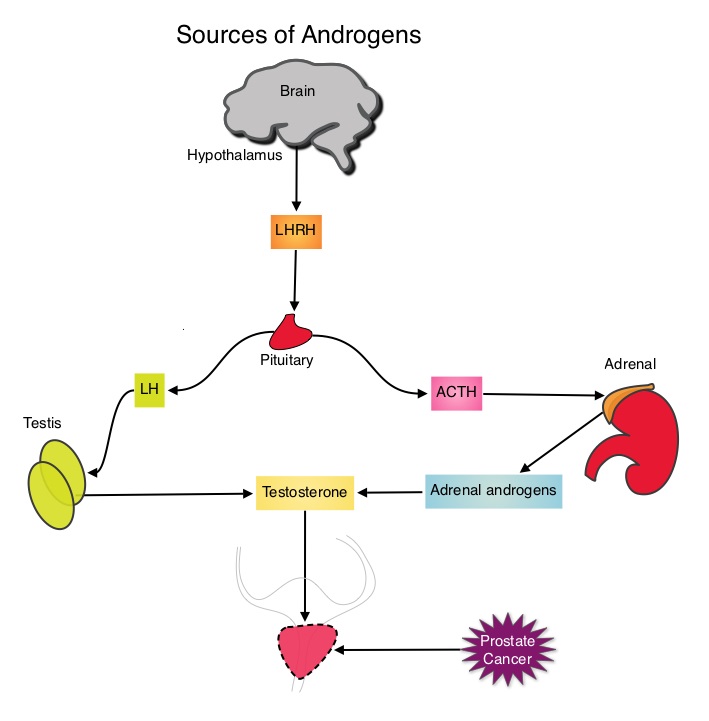 The development and progression of prostate cancer is driven by androgens. Androgen Deprivation Therapy (ADT) has therefore been the cornerstone of treatment of advanced prostate cancer and is the first treatment intervention for hormone sensitive prostate cancer. Chemotherapy is usually considered for patients who progress on hormone therapy and TAXOTERE® (Docetaxel) has been shown to improve Overall Survival (OS) of metastatic prostate cancer patients, who had progressed on androgen deprivation therapy. It is not clear however, whether ADT is more effective with or without TAXOTERE®, when treating patients with metastatic prostate cancer. To address this further, a randomized phase III trial (E3805) was conducted to assess the benefit of upfront treatment with a combination of chemotherapy and hormonal therapy, in patients with metastatic hormone sensitive prostate cancer. Seven hundred and ninety (N=790) patients with newly diagnosed metastatic prostate cancer were randomly assigned to receive either Androgen Deprivation Therapy alone (N=393) or ADT plus TAXOTERE® (N=397). Androgen Deprivation Therapy consisted of either Luteinizing Hormone Releasing Hormone (LHRH) agonist therapy, LHRH antagonist therapy, or surgical castration. Chemotherapy consisted of TAXOTERE®, started within 4 months of starting ADT, dosed at 75 mg/m2 given every 3 weeks for a maximum of six cycles. The median age of patients was 63 years and approximately two-thirds of patients had high-volume disease, with either extensive liver or bone metastases. The primary endpoint of this study was Overall Survival. At a median follow up of 29 months, the median Overall Survival was 42.3 months in the ADT group and 52.7 months in the ADT plus TAXOTERE® group (HR=0.63; P<0.0006). This benefit was even more significant in patients with high volume disease (32.2 vs 49.2 months for ADT and ADT plus TAXOTERE® respectively, HR=0.62; P<0.0012). At 12 months, the proportion of patients with PSA levels less than 0.2 ng/mL was 9.4% in the ADT alone group vs 19.7% in the ADT plus TAXOTERE® group (P < 0.0001). The median time to clinical progression was 19.8 months in the ADT alone group vs 32.7 months in the ADT plus TAXOTERE® group (P < 0.0001). The authors concluded that this is the first study to demonstrate survival benefit in patients with newly diagnosed metastatic prostate cancer. This survival benefit with Androgen Deprivation Therapy and TAXOTERE® is even more so, in patients with high volume disease and should be considered standard treatment for those patients who are fit to receive TAXOTERE® based therapy. Sweeney C, Chen Y, Carducci MA, et al. 2014 ASCO Annual Meeting; LBA2
The development and progression of prostate cancer is driven by androgens. Androgen Deprivation Therapy (ADT) has therefore been the cornerstone of treatment of advanced prostate cancer and is the first treatment intervention for hormone sensitive prostate cancer. Chemotherapy is usually considered for patients who progress on hormone therapy and TAXOTERE® (Docetaxel) has been shown to improve Overall Survival (OS) of metastatic prostate cancer patients, who had progressed on androgen deprivation therapy. It is not clear however, whether ADT is more effective with or without TAXOTERE®, when treating patients with metastatic prostate cancer. To address this further, a randomized phase III trial (E3805) was conducted to assess the benefit of upfront treatment with a combination of chemotherapy and hormonal therapy, in patients with metastatic hormone sensitive prostate cancer. Seven hundred and ninety (N=790) patients with newly diagnosed metastatic prostate cancer were randomly assigned to receive either Androgen Deprivation Therapy alone (N=393) or ADT plus TAXOTERE® (N=397). Androgen Deprivation Therapy consisted of either Luteinizing Hormone Releasing Hormone (LHRH) agonist therapy, LHRH antagonist therapy, or surgical castration. Chemotherapy consisted of TAXOTERE®, started within 4 months of starting ADT, dosed at 75 mg/m2 given every 3 weeks for a maximum of six cycles. The median age of patients was 63 years and approximately two-thirds of patients had high-volume disease, with either extensive liver or bone metastases. The primary endpoint of this study was Overall Survival. At a median follow up of 29 months, the median Overall Survival was 42.3 months in the ADT group and 52.7 months in the ADT plus TAXOTERE® group (HR=0.63; P<0.0006). This benefit was even more significant in patients with high volume disease (32.2 vs 49.2 months for ADT and ADT plus TAXOTERE® respectively, HR=0.62; P<0.0012). At 12 months, the proportion of patients with PSA levels less than 0.2 ng/mL was 9.4% in the ADT alone group vs 19.7% in the ADT plus TAXOTERE® group (P < 0.0001). The median time to clinical progression was 19.8 months in the ADT alone group vs 32.7 months in the ADT plus TAXOTERE® group (P < 0.0001). The authors concluded that this is the first study to demonstrate survival benefit in patients with newly diagnosed metastatic prostate cancer. This survival benefit with Androgen Deprivation Therapy and TAXOTERE® is even more so, in patients with high volume disease and should be considered standard treatment for those patients who are fit to receive TAXOTERE® based therapy. Sweeney C, Chen Y, Carducci MA, et al. 2014 ASCO Annual Meeting; LBA2
Confirmatory open-label, single-arm, multicenter phase 2 study of the BiTE antibody, Blinatumomab in patients (pts) with relapsed/refractory B-precursor acute lymphoblastic leukemia (r/r ALL)
SUMMARY: The FDA on December 3, 2014, granted accelerated approval to BLINCYTO® (Blinatumomab), a bispecific T cell engager (BiTE) antibody, for treatment of Philadelphia chromosome-negative (Ph-) Relapsed or Refractory B- cell precursor Acute Lymphoblastic Leukemia (ALL). BiTE® technology engages the body's immune system to detect and target malignant cells. These modified antibodies are designed to engage two different targets simultaneously, thereby placing the T cells within reach of the targeted cancer cell and facilitating apoptosis of the cancer cell. BiTE® antibodies are currently being investigated to treat a wide variety of malignancies.  BLINCYTO® (Blinatumomab) is an investigational BiTE® antibody designed to direct the patients T cells against CD19, a protein found on the surface of B-cell derived leukemias and lymphomas. The approval was based on a multicenter single-arm phase II trial in which 185 patients with Relapsed or Refractory Philadelphia chromosome negative ALL patients were enrolled. The median age was 39 years, and patients had their 1st relapse and were refractory to post hematopoietic stem cell transplantation less than 12 months before. About a third of the patients had at least 2 salvage therapies. BLINCYTO® was given by continuous IV infusion, 4 weeks on and 2 weeks off for up to 5 cycles and the median number of cycles given were 2. The primary endpoint was complete remission (CR) and response with a reduction in Minimal Residual Disease (MRD) to less than 10-4 or CR with partial hematological recovery (CRh), within the first 2 cycles of treatment. It was noted that 32% of patients attained CR with 2 cycles of treatment with BLINCYTO® and these responses were durable (median 6.7 months). Further, 31% of the patients in this study had a CR with or without complete hematological recovery but with reduction in MRD to less than 10-4. At the time of primary analysis, 80% of responses occurred within cycle 1. Further, the Complete Remissions (CR) and CR with partial hematological recovery (CRh) were seen in all subgroups of patients, although this was more pronounced in those with less than 50% bone marrow blasts. The median Relapse Free Survival and Overall survival were 5.9 months and 6.1 months respectively. The most frequent grade 3 adverse events were febrile neutropenia, neutropenia and anemia, occurring in 26%, 15% and 15% of patients, respectively. The authors concluded that BLINCYTO® has significant single agent antileukemia activity in a difficult-to-treat population with Relapsed and Refractory Acute Lymphoblastic Leukemia. Future studies will hopefully address whether BLINCYTO® can serve as a bridge to transplantation, in patients with Relapsed and Refractory B-cell ALL. Cytokine Release Syndrome can result from the activation of the immune system. The FDA approved BLINCYTO® with a Risk Evaluation and Mitigation Strategy (REMS). Topp MS, Goekbuget N, Stein AS, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 7005)
BLINCYTO® (Blinatumomab) is an investigational BiTE® antibody designed to direct the patients T cells against CD19, a protein found on the surface of B-cell derived leukemias and lymphomas. The approval was based on a multicenter single-arm phase II trial in which 185 patients with Relapsed or Refractory Philadelphia chromosome negative ALL patients were enrolled. The median age was 39 years, and patients had their 1st relapse and were refractory to post hematopoietic stem cell transplantation less than 12 months before. About a third of the patients had at least 2 salvage therapies. BLINCYTO® was given by continuous IV infusion, 4 weeks on and 2 weeks off for up to 5 cycles and the median number of cycles given were 2. The primary endpoint was complete remission (CR) and response with a reduction in Minimal Residual Disease (MRD) to less than 10-4 or CR with partial hematological recovery (CRh), within the first 2 cycles of treatment. It was noted that 32% of patients attained CR with 2 cycles of treatment with BLINCYTO® and these responses were durable (median 6.7 months). Further, 31% of the patients in this study had a CR with or without complete hematological recovery but with reduction in MRD to less than 10-4. At the time of primary analysis, 80% of responses occurred within cycle 1. Further, the Complete Remissions (CR) and CR with partial hematological recovery (CRh) were seen in all subgroups of patients, although this was more pronounced in those with less than 50% bone marrow blasts. The median Relapse Free Survival and Overall survival were 5.9 months and 6.1 months respectively. The most frequent grade 3 adverse events were febrile neutropenia, neutropenia and anemia, occurring in 26%, 15% and 15% of patients, respectively. The authors concluded that BLINCYTO® has significant single agent antileukemia activity in a difficult-to-treat population with Relapsed and Refractory Acute Lymphoblastic Leukemia. Future studies will hopefully address whether BLINCYTO® can serve as a bridge to transplantation, in patients with Relapsed and Refractory B-cell ALL. Cytokine Release Syndrome can result from the activation of the immune system. The FDA approved BLINCYTO® with a Risk Evaluation and Mitigation Strategy (REMS). Topp MS, Goekbuget N, Stein AS, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 7005)
