Biomarkers May Predict Response to BRAF and MEK inhibitors in Malignant Melanoma
SUMMARY: It is estimated that in the US, approximately 100,350 new cases of malignant melanoma will be diagnosed in 2020 and about 6850 patients are expected to die of the disease. The incidence of melanoma has been on the rise for the past three decades. Surgical resection with a curative intent is the standard of care for patients with early stage melanoma, with a 5-year survival rate of 98% for Stage I disease and 90% for Stage II disease. Patients with locally advanced or metastatic melanoma historically have had poor outcomes. With the development and availability of immune checkpoint inhibitors and BRAF and MEK inhibitors, this patient group now has significantly improved outcomes. In treatment naïve patients receiving anti-PD-1 therapies such as KEYTRUDA® (Pembrolizumab) or OPDIVO® (Nivolumab) in Phase III trials, the Progression Free Survival (PFS) rates have ranged from 27-31%, with an Overall Survival (OS) rate of 46% at 4 years. The 5-year OS among patients receiving KEYTRUDA® was 43%, and in those treated with a combination of OPDIVO® plus YERVOY® (Ipilimumab), 4-year PFS and OS rates were 37% and 53%, respectively.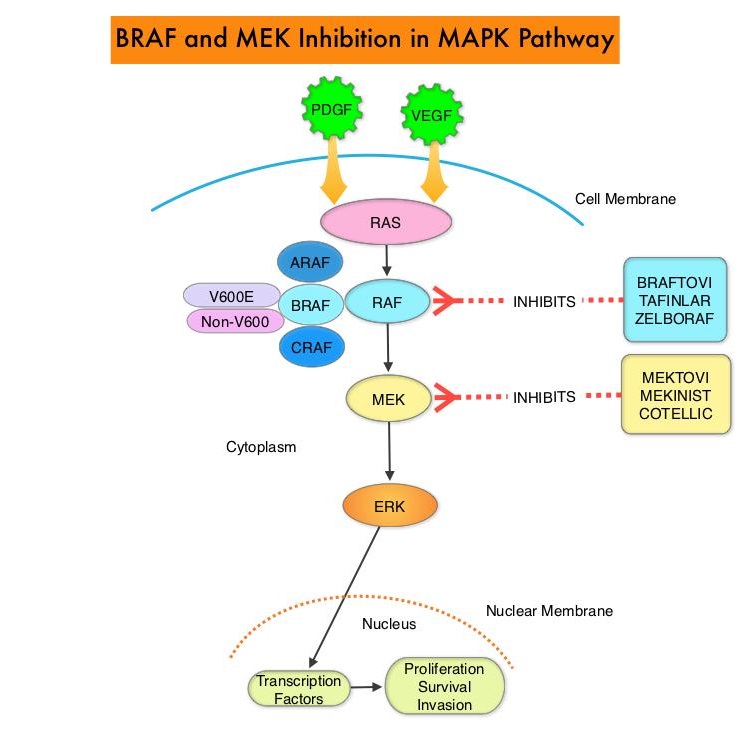
The Mitogen-Activated Protein Kinase pathway (MAPK pathway) is an important signaling pathway which enables the cell to respond to external stimuli. This pathway plays a dual role, regulating cytokine production and participating in cytokine dependent signaling cascade. The MAPK pathway of interest is the RAS-RAF-MEK-ERK pathway. The RAF family of kinases includes ARAF, BRAF and CRAF signaling molecules. BRAF is a very important intermediary of the RAS-RAF-MEK-ERK pathway. BRAF mutations have been detected in 6-8% of all malignancies. The most common BRAF mutation in melanoma is at the V600E/K site and is detected in approximately 50% of melanomas, and result in constitutive activation of the MAPK pathway.
TAFINLAR® (Dabrafenib), is a selective oral BRAF inhibitor and MEKINIST® (Trametinib) is a potent and selective inhibitor of MEK gene, which is downstream from RAF in the MAPK pathway. Long term survival data pooled from two randomized Phase III COMBI-d and COMBI-v trials, which involved previously untreated, unresectable or metastatic melanoma patients, with BRAFV600E or V600K mutation who had received TAFINLAR® along with MEKINIST® showed PFS rates of 21% at 4 years and 19% at 5 years. The OS rates were 37% at 4 years and 34% at 5 years. The 5-year OS rate was 71% among patients who had a Complete Response and 55% among those who had a normal LDH level plus fewer than three metastatic organ sites at baseline.
With the approval of multiple therapeutic options for the management of patients with BRAF-mutant melanoma, treatment decisions have become increasingly complex. In patients with limited disease burden, immunotherapy with checkpoint inhibitors is favored by most clinicians based on the long term data supporting the durability of responses with immunotherapies. On the contrary, BRAF-targeted agents are utilized in patients with extensive, symptomatic disease and active brain metastases. The optimal sequence of these therapeutic strategies in order to improve long-term patient outcome, has remained unclear.
COMBI-AD is an international, multi-center, randomized, double-blind, placebo-controlled, Phase III trial, in which 870 patients with completely resected, Stage III melanoma and with BRAF V600E or V600K mutations were enrolled. Patients were randomly assigned in a 1:1 to receive TAFINLAR® 150 mg orally twice daily in combination with MEKINIST® 2 mg orally once daily (N=438) or two matched placebos (N=432). Treatment was given for 12 months. At a median follow up of 2.8 years, the estimated 3-year Relapse Free Survival (RFS) rate was 58% with a combination of TAFINLAR® and MEKINIST® and 39% in the placebo group (HR=0.47; P<0.001), and this represented a 53% lower risk of relapse. The risk of distant metastases or death was reduced by 49% with the combination therapy versus placebo (HR=0.51; P<0.001). A prespecified exploratory outcome of this trial was assessment of biomarkers. The authors assessed intrinsic tumor genomic features in 368 patients using Next-Generation DNA sequencing, and tumor microenvironment characteristics were assessed in 507 patients by use of a NanoString RNA assay, in an attempt to provide prognostic and predictive information. Median follow up at data cutoff was 44 months in the TAFINLAR® plus MEKINIST® group and 42 months in the placebo group.
Baseline MAPK pathway genomic alterations did not affect treatment benefit or outcomes in either treatment groups. An Interferon Gamma gene expression signature higher than the median was prognostic for prolonged RFS in both treatment groups. Tumor Mutational Burden (TMB) was independently associated with better RFS in the placebo group (HR for top third versus bottom third of TMB values=0.56; P=0.0056), but this benefit was not seen in the TAFINLAR® plus MEKINIST® group (HR= 0.83; P=0.44). However, patients with TMB in the lower two terciles who received TAFINLAR® plus MEKINIST® combination had improved RFS compared to those who received placebo (HR=0.49: P<0.0001). Patients with high TMB appeared to have a less pronounced benefit with TAFINLAR® plus MEKINIST® targeted therapy, compared to placebo, especially if they had an Interferon Gamma gene expression signature lower than the median.
It was concluded from this biomarker analysis that high Tumor Mutational Burden was independently associated with better Relapse Free Survival in the placebo group but not in the TAFINLAR® plus MEKINIST® combination group, and an Interferon Gamma gene expression signature higher than the median was prognostic for prolonged RFS in both treatment groups. Adjuvant dabrafenib plus trametinib versus placebo in patients with resected, BRAFV600-mutant, stage III melanoma (COMBI-AD): exploratory biomarker analyses from a randomised, phase 3 trial. Dummer R, Brase JC, Garrett J, et al. The Lancet Oncology. Published:January 30, 2020DOI:https://doi.org/10.1016/S1470-2045(20)30062-0

