SUMMARY: The American Cancer Society's estimates that approximately 15,720 new cases of chronic lymphocytic leukemia (CLL) will be diagnosed in 2014 and approximately 4600 patients will die from the disease. CLL is a disease of the elderly and the average age at the time of diagnosis is 72 years. Majority of these patients have associated comorbidities and would be considered inappropriate for Fludarabine (FLUDARA®) based therapy. COMPLEMENT 1 is a randomized, open-label, multicenter, phase III trial in which Ofatumumab (ARZERRA®) in combination with Chlorambucil (LEUKERAN®) was compared to single agent LEUKERAN®. ARZERRA® is a second generation fully human IgG 1 monoclonal antibody. Unlike Rituximab (RITUXAN®) which is the chimeric monoclonal antibody, ARZERRA® targets a different region (different epitope) of the CD20 molecule. To go back to basics, several antigen molecules are expressed on the surface of normal B cells. Majority of these antigens are involved in cell growth, proliferation, differentiation, immune regulation and complement activation. The various stages of B cell development include hematopoietic stem cell, lymphoid stem cell, Pro B cell, Pre B cell, Immature B cell and Mature B-cell, Activated B cell, Memory B cell and Plasma cell. The CD20 molecule is expressed at specific stages of B cell development (Pre B cell stage to Mature B lymphocyte stage) and on malignant B cells. This molecule however is not expressed on hematopoietic stem cells and plasma cells. As such, targeting CD20 with therapeutic monoclonal antibodies spares the Pro B cell which is a precursor of Pre B cell and this along with intact hematopoietic stem cell facilitates post treatment recovery of B cells. As the plasma cells are spared as well, serum IgG levels are maintained. Monoclonal antibodies targeting CD20 destroy CD20 positive B cells by 3 different mechanisms. They include Antibody Dependent Cellular Cytotoxicity (ADCC), Complement Dependent Cytotoxicity (CDC) and programmed cell death (Apoptosis).
Monoclonal antibodies targeting CD20 destroy CD20 positive B cells by 3 different mechanisms. They include Antibody Dependent Cellular Cytotoxicity (ADCC), Complement Dependent Cytotoxicity (CDC) and programmed cell death (Apoptosis).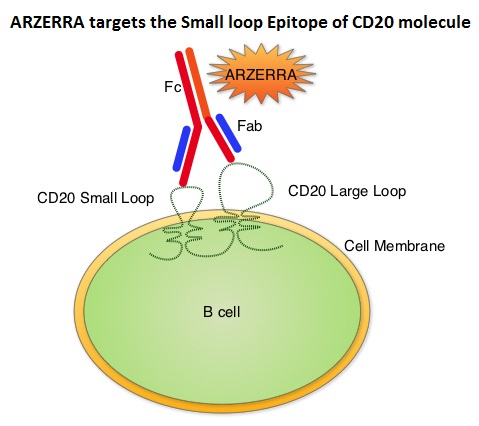 Unlike RITUXAN®, ARZERRA® targets the Small loop epitope of CD20 molecule which is proximal to the B cell membrane and this has been shown to be associated with highly efficient cell lysis through complement dependent cytotoxicity. So, compared to RITUXAN®. ARZERRA® has stronger CDC, similar ADCC and does not appear to induce Apoptosis. In this study, 447 CLL patients for whom FLUDARA® based therapy was considered to be inappropriate due to age and comorbidities, were randomly assigned 1:1 to receive either ARZERRA® in combination with LEUKERAN® or LEUKERAN® alone. ARZERRA® was given as an IV infusion at a dose of 300 mg on Cycle 1, Day 1, 1000 mg on Cycle 1, Day 8 and 1000 mg administered on Day 1 of all subsequent 28 day cycles. LEUKERAN® was given at a dose of 10 mg/m2 orally on Days 1 to 7 every 28 days in both treatment groups. The median age was 69 years and majority of the patients had 2 or more comorbidities. The primary endpoint of this study was Progression Free Survival (PFS) and secondary endpoints included Overall Response Rate (ORR), Overall Survival (OS) and safety. The median number of cycles in both treatment groups was 6. The median PFS was 22.4 months for patients receiving ARZERRA® in combination with LEUKERAN® compared with 13.1 months for those receiving single agent LEUKERAN® (HR=0.57, P< 0.001). The ORR was higher with the combination regimen versus single agent LEUKERAN® (82% vs 69%, P=0.001) and 37% of patients in the combination arm were MRD negative. The median OS for the combination group was not reached. The majority of adverse reactions were Grade 2 or lower, in both of the treatment arms and included infusion reactions, neutropenia, asthenia, headache, herpes simplex, lower respiratory tract infections, arthralgia and upper abdominal pain. The authors concluded that ARZERRA® in combination with LEUKERAN® is a clinically important milestone, in the management of elderly patients with CLL, who are considered inappropriate for FLUDARA® based therapy. Hillmen P, Robak T, Janssens A, et al. Blood 2013;122: Abstract#528
Unlike RITUXAN®, ARZERRA® targets the Small loop epitope of CD20 molecule which is proximal to the B cell membrane and this has been shown to be associated with highly efficient cell lysis through complement dependent cytotoxicity. So, compared to RITUXAN®. ARZERRA® has stronger CDC, similar ADCC and does not appear to induce Apoptosis. In this study, 447 CLL patients for whom FLUDARA® based therapy was considered to be inappropriate due to age and comorbidities, were randomly assigned 1:1 to receive either ARZERRA® in combination with LEUKERAN® or LEUKERAN® alone. ARZERRA® was given as an IV infusion at a dose of 300 mg on Cycle 1, Day 1, 1000 mg on Cycle 1, Day 8 and 1000 mg administered on Day 1 of all subsequent 28 day cycles. LEUKERAN® was given at a dose of 10 mg/m2 orally on Days 1 to 7 every 28 days in both treatment groups. The median age was 69 years and majority of the patients had 2 or more comorbidities. The primary endpoint of this study was Progression Free Survival (PFS) and secondary endpoints included Overall Response Rate (ORR), Overall Survival (OS) and safety. The median number of cycles in both treatment groups was 6. The median PFS was 22.4 months for patients receiving ARZERRA® in combination with LEUKERAN® compared with 13.1 months for those receiving single agent LEUKERAN® (HR=0.57, P< 0.001). The ORR was higher with the combination regimen versus single agent LEUKERAN® (82% vs 69%, P=0.001) and 37% of patients in the combination arm were MRD negative. The median OS for the combination group was not reached. The majority of adverse reactions were Grade 2 or lower, in both of the treatment arms and included infusion reactions, neutropenia, asthenia, headache, herpes simplex, lower respiratory tract infections, arthralgia and upper abdominal pain. The authors concluded that ARZERRA® in combination with LEUKERAN® is a clinically important milestone, in the management of elderly patients with CLL, who are considered inappropriate for FLUDARA® based therapy. Hillmen P, Robak T, Janssens A, et al. Blood 2013;122: Abstract#528
Author: RR
ARZERRA® (Ofatumumab)
ARZERRA® (Ofatumumab): The FDA on April 17, 2014 approved ARZERRA® in combination with LEUKERAN® (Chlorambucil), for the treatment of previously untreated patients with Chronic Lymphocytic Leukemia (CLL), for whom FLUDARA® (Fludarabine) based therapy is considered inappropriate. ARZERRA® first received accelerated approval in 2009, for the treatment of patients with CLL, refractory to FLUDARA® and CAMPATH® (Alemtuzumab). ARZERRA® injection is given as an intravenous infusion and is a product GlaxoSmithKline.
ZYTIGA® improves survival without impacting Quality of Life in CRPC
In a recent article published in The Lancet Oncology, ZYTIGA® (Abiraterone) given along with prednisone delayed patient-reported pain progression and deterioration of Quality of Life in chemotherapy-naive patients with metastatic Castrate Resistant Prostate Cancer (CRPC). This was accomplished without compromising efficacy, which was survival benefit. This is relevant because, patients with Prostate Cancer in general are elderly and it is important that any treatment intervention in this patient population with asymptomatic or mildly symptomatic CRPC improves overall survival without negatively impacting Quality of Life.
Neoadjuvant therapy for rectal cancer Mature results from NSABP protocol R-04
SUMMARY: The American Cancer Society's estimates 40,000 new cases of rectal cancer in the United States for 2014. Rectal cancer diagnosed at an early stage such as Stage II (T3-T4, N0) or Stage III (Node positive disease without distant metastases) is potentially curable with a combination of neoadjuvant (preoperative) chemoradiation, surgery and postoperative chemotherapy. Unlike colon cancer, the risk of locoregional recurrence is high in rectal cancer due to its close proximity to the surrounding pelvic organs and difficulty in obtaining a clear surgical margins. Further, there is no serosal tissue surrounding the rectum. For all these reasons, preoperative Radiation Therapy (RT) with concurrent fluoropyrimidine based chemotherapy as a radiosensitizer followed by postoperative chemotherapy (total of 6 months of perioperative chemotherapy) has been the standard intervention. Infusional 5 Fluorouracil (5-FU) is often incorporated with concurrent radiation. This however is cumbersome and inconvenient for the patients. The NSABP protocol R-04 trial is a four arm phase III trial in which 1608 patients with clinical stage II or III rectal cancer undergoing preoperative RT were randomly assigned to one of four chemotherapy regimens – Continuous Infusion (CI) 5-FU 225mg/m2 over 24 hours, 5 days a week x 5 weeks (N=477), CI 5-FU with IV Oxaliplatin (ELOXATIN®) 50mg/m2 /wk x 5 weeks (N=329), Capecitabine (XELODA®) 825 mg/m2 PO BID 5 days/wk x 5 weeks (N=472) or XELODA® with ELOXATIN®, x 5 weeks (N=330). Radiation therapy consisted of 4,500cGy in 25 fractions over 5 wks plus a boost. The primary goals of this study were to compare preoperative XELODA® and CI 5-FU given along with concurrent pelvic RT and also determine whether ELOXATIN® would be of additional benefit. The primary endpoint of local-regional tumor control included locoregional tumor recurrence, less than complete surgical resection and no surgery. When combined with RT, this study showed no significant differences in local-regional tumor control, Disease Free Survival or Overall Survival between infusional 5-FU and XELODA® given alone or in combination with ELOXATIN®. The addition of ELOXATIN® was however associated with significantly more grade 3-4 diarrhea (P<0.0001). The authors concluded that outcomes and toxicities are similar with CI 5-FU or oral XELODA® when combined with RT. The addition of ELOXATIN® did not improve outcomes but resulted in significant toxicity. Oral XELODA® therefore obviates the need for central venous access and ambulatory infusion pumps and makes it more convenient for the patients without compromising efficacy. Allegra CJ, Yothers G, O'Connell MJ, et al. J Clin Oncol 32, 2014 (suppl 3; abstr 390)
Abiraterone acetate plus prednisone versus prednisone alone in chemotherapy-naive men with metastatic castration-resistant prostate cancer patient-reported outcome results of a randomised phase 3 trial
SUMMARY: Prostate Cancer is the most common cancer in American men and approximately 233,000 new cases will be diagnosed in 2014 and close to 30,000 men will die of the disease. The primary systemic intervention for patients with advanced prostate cancer is Androgen Deprivation Therapy (ADT). This can be accomplished by either surgical castration (bilateral orchicetomy) or medical castration, using LHRH (GnRH- Gonadotropin-Releasing Hormone) agonists. Majority of these patients will eventually develop progressive disease (Castrate Resistant Prostate cancer – CRPC), due to enhanced autocrine and /or paracrine synthesis of androgens or androgen precursors in the tumor micro environment. This has lead to the development of novel compounds that decrease androgen synthesis as well as androgen signaling in patients with CRPC.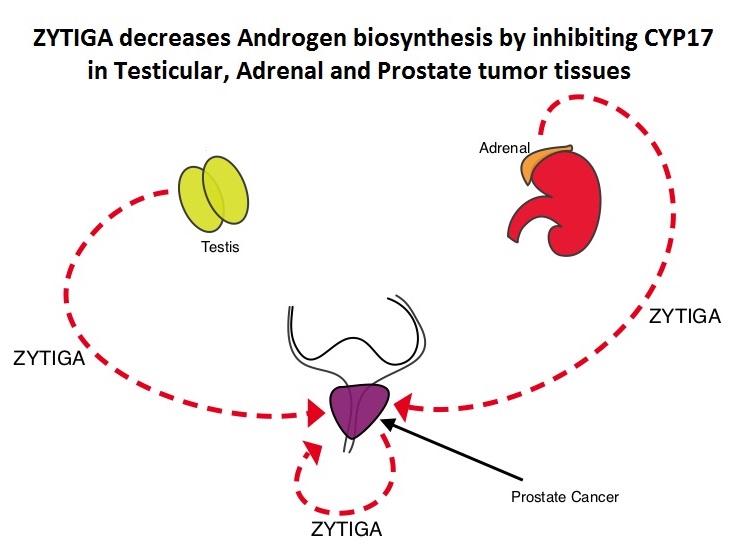 Abiraterone acetate (ZYTIGA®) is a novel, targeted, oral androgen biosynthesis inhibitor that decreases androgen production in the adrenal glands, testes and prostate cancer cells by inhibiting a steroidal enzyme CYP17A. Phase III trials have demonstrated that treatment with ZYTIGA® prolongs overall survival in metastatic CRPC patients, who had progressed after TAXOTERE® (Docetaxel) therapy, as well as those who are chemotherapy naive. ZYTIGA® delays deterioration of performance status, progression of fatigue and pain as well as development of skeletal related events, in TAXOTERE® refractory patients. It is important that any treatment considered for patients with asymptomatic or mildly symptomatic CRPC improves overall survival without negatively impacting Quality of Life. To address this further, the authors analyzed patient reported data related to pain and Quality of Life from a large randomized clinical trial. Of the 1088 chemotherapy-naïve, asymptomatic or mildly symptomatic CRPC patients randomized in this double-blind study, 546 patients received ZYTIGA® 1000 mg PO plus prednisone 5 mg twice daily and 542 patients received placebo plus prednisone. At the time of the planned interim analysis, ZYTIGA® improved radiographic progression-free survival, overall survival, and significantly delayed the initiation of chemotherapy. The authors in this publication reported the data related to pain and Quality of Life of these patients, at the time of the second preplanned interim analysis. Pain was assessed with the Brief Pain Inventory-Short Form (BPI-SF) questionnaire, which is a validated instrument to assess pain and Health Related Quality of Life (HRQoL) was measured with the Functional Assessment of Cancer Therapy—Prostate (FACT-P) questionnaire, which is a validated tool for metastatic CRPC. At a median follow-up of 22.2 months, the median time to progression of pain intensity was longer in patients receiving ZYTIGA® plus prednisone vs placebo plus prednisone (26.7 months vs 18.4 months, HR=0.82, P=0.049). The median time for pain to progress and interfere with daily activities was 10.3 months for ZYTIGA® vs 7.4 months for placebo (HR= 0.79, P=0.005). The median time to deterioration of HRQoL was longer in patients receiving ZYTIGA® plus prednisone vs those receiving placebo plus prednisone, as assessed by the FACT-P total score (12.7 months vs 8.3 months, HR=0.78, P=0.003). The authors concluded that ZYTIGA® given along with prednisone delays patient-reported pain progression and deterioration of HRQol, in chemotherapy-naive patients with metastatic CRPC, without compromising efficacy. Basch E, Autio K, Ryan CJ, et al. The Lancet Oncology 2013;14:1193 -1199
Abiraterone acetate (ZYTIGA®) is a novel, targeted, oral androgen biosynthesis inhibitor that decreases androgen production in the adrenal glands, testes and prostate cancer cells by inhibiting a steroidal enzyme CYP17A. Phase III trials have demonstrated that treatment with ZYTIGA® prolongs overall survival in metastatic CRPC patients, who had progressed after TAXOTERE® (Docetaxel) therapy, as well as those who are chemotherapy naive. ZYTIGA® delays deterioration of performance status, progression of fatigue and pain as well as development of skeletal related events, in TAXOTERE® refractory patients. It is important that any treatment considered for patients with asymptomatic or mildly symptomatic CRPC improves overall survival without negatively impacting Quality of Life. To address this further, the authors analyzed patient reported data related to pain and Quality of Life from a large randomized clinical trial. Of the 1088 chemotherapy-naïve, asymptomatic or mildly symptomatic CRPC patients randomized in this double-blind study, 546 patients received ZYTIGA® 1000 mg PO plus prednisone 5 mg twice daily and 542 patients received placebo plus prednisone. At the time of the planned interim analysis, ZYTIGA® improved radiographic progression-free survival, overall survival, and significantly delayed the initiation of chemotherapy. The authors in this publication reported the data related to pain and Quality of Life of these patients, at the time of the second preplanned interim analysis. Pain was assessed with the Brief Pain Inventory-Short Form (BPI-SF) questionnaire, which is a validated instrument to assess pain and Health Related Quality of Life (HRQoL) was measured with the Functional Assessment of Cancer Therapy—Prostate (FACT-P) questionnaire, which is a validated tool for metastatic CRPC. At a median follow-up of 22.2 months, the median time to progression of pain intensity was longer in patients receiving ZYTIGA® plus prednisone vs placebo plus prednisone (26.7 months vs 18.4 months, HR=0.82, P=0.049). The median time for pain to progress and interfere with daily activities was 10.3 months for ZYTIGA® vs 7.4 months for placebo (HR= 0.79, P=0.005). The median time to deterioration of HRQoL was longer in patients receiving ZYTIGA® plus prednisone vs those receiving placebo plus prednisone, as assessed by the FACT-P total score (12.7 months vs 8.3 months, HR=0.78, P=0.003). The authors concluded that ZYTIGA® given along with prednisone delays patient-reported pain progression and deterioration of HRQol, in chemotherapy-naive patients with metastatic CRPC, without compromising efficacy. Basch E, Autio K, Ryan CJ, et al. The Lancet Oncology 2013;14:1193 -1199
Rapid Increase in Breast Magnetic Resonance Imaging Use Trends From 2000 to 2011
SUMMARY: Breast cancer is the most common cancer among women in the United States and 1 in 8 women will develop invasive breast cancer during their lifetime. Screening mammography complemented by breast self exam and clinical breast exam has resulted in early detection of breast cancer and successful outcomes. Even though mammography is a sensitive screening test, a small percentage of breast cancers may not show up on mammograms but may be palpable on examination by the patient or the clinician. Further, mammograms are less likely to find breast tumors in younger women with dense breast tissue. A breast Magnetic Resonance Imaging (MRI) is more sensitive than mammography although the specificity of a breast MRI is lower, resulting in a higher rate of false-positive findings and potentially unnecessary biopsies. Microcalcifications in the breast can be missed by a breast MRI. Taking these factors into consideration, appropriate utilization of breast MRI becomes relevant.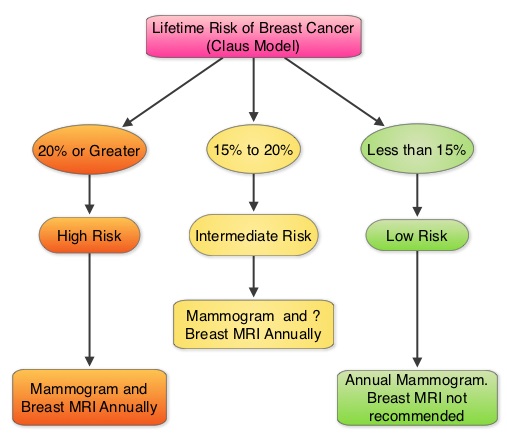
 The American Cancer Society (ACS) recommends an annual MRI as an adjunct to screening mammogram and clinical breast exam in certain groups with increased risk of breast cancer (See Figures). The authors conducted this study to determine the utilization of breast (MRI) in community settings. In this retrospective study, the authors reviewed data on 10,518 women from a not-for-profit health plan, and these women had at least one breast MRI between January 2000 and December 2011. The appropriateness of a breast MRI was determined using a prediction model obtained from electronic medical records on a subset of patients. Between 2000 and 2009, there was a 20-fold increase in the use of breast MRI from 6.5 per 10,000 women in the year 2000 to 130.7 per 10,000 in 2009. This increase then declined and stabilized to 104.8 per 10,000 by 2011. With regards to indications for a breast MRI, 51.7% had a family history of breast cancer, 30.1% had a personal history of breast cancer and 3.5% of women had a documented genetic mutation. It is interesting to note that of those who received screening or surveillance breast MRI’s, only 21% fulfilled the American Cancer Society (ACS) criteria for a breast MRI. Conversely, fewer participants (48.4%), with documented deleterious genetic mutations, received breast MRI screening. The authors concluded that breast MRI was over utilized in those who did not fit the ACS criteria and was under utilized in those with documented genetic mutations. It is their opinion that routine breast MRI screening is not recommended for a new breast cancer diagnosis or for breast cancer surveillance and should only be considered for the group of individuals who have the most benefit. Breast MRI is performed preferably between days 7-15 of menstrual cycle for premenopausal women, using a dedicated breast coil, with the ability to perform a biopsy under MRI guidance by experienced radiologists, during the same visit. Stout NK, Nekhlyudov L, Li L, et al. JAMA Intern Med. 2014;174:114-121.
The American Cancer Society (ACS) recommends an annual MRI as an adjunct to screening mammogram and clinical breast exam in certain groups with increased risk of breast cancer (See Figures). The authors conducted this study to determine the utilization of breast (MRI) in community settings. In this retrospective study, the authors reviewed data on 10,518 women from a not-for-profit health plan, and these women had at least one breast MRI between January 2000 and December 2011. The appropriateness of a breast MRI was determined using a prediction model obtained from electronic medical records on a subset of patients. Between 2000 and 2009, there was a 20-fold increase in the use of breast MRI from 6.5 per 10,000 women in the year 2000 to 130.7 per 10,000 in 2009. This increase then declined and stabilized to 104.8 per 10,000 by 2011. With regards to indications for a breast MRI, 51.7% had a family history of breast cancer, 30.1% had a personal history of breast cancer and 3.5% of women had a documented genetic mutation. It is interesting to note that of those who received screening or surveillance breast MRI’s, only 21% fulfilled the American Cancer Society (ACS) criteria for a breast MRI. Conversely, fewer participants (48.4%), with documented deleterious genetic mutations, received breast MRI screening. The authors concluded that breast MRI was over utilized in those who did not fit the ACS criteria and was under utilized in those with documented genetic mutations. It is their opinion that routine breast MRI screening is not recommended for a new breast cancer diagnosis or for breast cancer surveillance and should only be considered for the group of individuals who have the most benefit. Breast MRI is performed preferably between days 7-15 of menstrual cycle for premenopausal women, using a dedicated breast coil, with the ability to perform a biopsy under MRI guidance by experienced radiologists, during the same visit. Stout NK, Nekhlyudov L, Li L, et al. JAMA Intern Med. 2014;174:114-121.

Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17) final results of a randomised, phase 2/3 trial
SUMMARY: It is estimated that in 2014, over 18,000 new cases of esophageal carcinoma will be diagnosed in the U.S. and over 15,000 will die of the disease. There has been a rise in the incidence of esophageal cancer in recent decades. Even though stage 0, I, and II and most stage III esophageal cancers are potentially resectable, a significant number of these patients have associated comorbid conditions that may preclude them from undergoing surgical intervention. In this group of patients, as well as those with inoperable esophageal cancer, cisplatin based chemoradiation treatment has been associated with improved survival. Cisplatin however is associated with significant toxicities. The rationale for substituting ELOXATIN® for Cisplatin is based on its lower emetogenicity, lack of nephrotoxicity and lack of need for IV hydration. Prior phase II trials have demonstrated that ELOXATIN® (Oxaliplatin) based chemotherapy given alone or in combination with radiation therapy improved response rates with acceptable toxicities, in patients with advanced esophageal cancer. Based on this information, the authors conducted an open labeled, multicenter study to assess the efficacy and safety of the FOLFOX chemotherapy regimen (Fluorouracil plus Leucovorin and Oxaliplatin) compared to Fluorouracil and Cisplatin, when administered as a part of chemoradiotherapy treatment, in patients with locally advanced esophageal cancer. Patients with stage I—IVA esophageal carcinoma (adenocarcinoma, squamous-cell, or adenosquamous) who were not candidates for surgical intervention were randomly assigned to receive FOLFOX chemotherapy (N=131) or Cisplatin and Fluorouracil (N=128), with concurrent radiation. FOLFOX chemotherapy consisted of 6 cycles of ELOXATIN® 85 mg/m2 IV, Leucovorin 200 mg/m2 IV, Fluorouracil 400 mg/m2 IV bolus given on day 1, and infusional Fluorouracil 1600 mg/m2 given over 46 hours, every 2 weeks, with the first 3 cycles given concurrently with radiation therapy. Chemotherapy with Cisplatin and Fluorouracil consisted of 4 cycles of Cisplatin 75 mg/m2 IV given on day 1 and infusional Fluorouracil 1000 mg/m2 per day, given for 4 days, with the first 2 cycles given concurrently with radiation therapy at 4 week intervals and the remaining 2 cycles given 3 weeks apart after completion of radiation therapy. Both treatment groups received 50Gy radiotherapy, in 25 fractions, at five fractions per week. The primary endpoint was Progression Free Survival (PFS). At a median follow up of 25.3 months, the median PFS was 9.7 months in the FOLFOX group and 9.4 months in the Cisplatin group and this was not statistically significant (P=0.64), suggesting that FOLFOX in combination with radiation may be an alternative to Cisplatin and Fluorouracil. FOLFOX chemotherapy may alleviate Cisplatin related toxicities such as nausea and vomiting, nephrotoxicity and the need for IV hydration, making this a much more tolerable regimen in advanced esophageal carcinoma. Conroy T, Galais M, Raoul J, et al. Lancet Oncol, 2014;15: 305-314
Obinutuzumab plus Chlorambucil in Patients with CLL and Coexisting Conditions
SUMMARY: Chronic Lymphocytic leukemia (CLL) is a disease of the elderly with a median age at diagnosis of 72 years. Given the age at diagnosis, it is not uncommon for these patients to have multiple comorbidities. The authors in this trial attempted to study a new agent, Obinutuzumab or GAZYVA® (GA101) specifically in this patient population.  GAZYVA® is glycoengineered, fully humanized, third generation, type II anti-CD20 antibody (IgG1 monoclonal antibody) that selectivity binds to the extracellular domain of the CD20 antigen on malignant human B cells. By virtue of binding affinity of the glycoengineered Fc portion of GAZYVA® to Fcγ receptor III on innate immune effector cells such as natural killer cells, macrophages and neutrophils, antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis is significantly enhanced, whereas it induces very little complement-dependent cytotoxicity. This is in contrast to RITUXAN® (Rituximab), which is a first generation type I, chimeric anti-CD20 targeted monoclonal antibody that kills CLL cells primarily by complement-dependent cytotoxicity and also ADCC. In this phase III trial, LEUKERAN® (Chlorambucil) was compared with a combination of GAZYVA® plus LEUKERAN® and a combination of RITUXAN® plus LEUKERAN®. Five Hundred and eighty nine (589) treatment naïve CLL patients over 70 years of age with comorbidities were enrolled of whom 118 patients received LEUKERAN® alone, 238 received GAZYVA® plus LEUKERAN® and 233 received RITUXAN® plus LEUKERAN®. The primary endpoint was Progression-Free Survival (PFS). Chemoimmunotherapy with both GAZYVA® plus LEUKERAN® and RITUXAN® plus LEUKERAN® significantly prolonged PFS compared to LEUKERAN® alone. The median PFS was 11.1 months with LEUKERAN® alone compared to 26.7 months for GAZYVA® plus LEUKERAN® (HR=0.18, P<0.001) and 16.3 months for RITUXAN® plus LEUKERAN® (HR=0.44, P<0.001). This benefit was seen in all subgroups except those with del(17) and quality of life in those who received antibody along with LEUKERAN® was not compromised. The combination of GAZYVA® and LEUKERAN®, also prolonged overall survival when compared to LEUKERAN® alone (HR=0.41; P=0.002). This benefit however was not noted with the RITUXAN® plus LEUKERAN® combination. Treatment with GAZYVA® plus LEUKERAN® when compared with RITUXAN® plus LEUKERAN®, resulted in a longer PFS (26.7 vs15.2 months; HR=0.39; P<0.001), higher complete response rates (20.7% vs. 7.0%) and deeper molecular responses. Infusion related reactions were more common in the GAZYVA® plus LEUKERAN® group without increase in the risk for infections. The authors concluded that a combination of GAZYVA® and LEUKERAN® when given to elderly patients with comorbid conditions improved overall survival compared to LEUKERAN® alone and resulted in higher response rates and longer PFS than RITUXAN® plus LEUKERAN®. Goede V, Fischer K, Busch R, et al. N Engl J Med 2014; 370:1101-1110
GAZYVA® is glycoengineered, fully humanized, third generation, type II anti-CD20 antibody (IgG1 monoclonal antibody) that selectivity binds to the extracellular domain of the CD20 antigen on malignant human B cells. By virtue of binding affinity of the glycoengineered Fc portion of GAZYVA® to Fcγ receptor III on innate immune effector cells such as natural killer cells, macrophages and neutrophils, antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis is significantly enhanced, whereas it induces very little complement-dependent cytotoxicity. This is in contrast to RITUXAN® (Rituximab), which is a first generation type I, chimeric anti-CD20 targeted monoclonal antibody that kills CLL cells primarily by complement-dependent cytotoxicity and also ADCC. In this phase III trial, LEUKERAN® (Chlorambucil) was compared with a combination of GAZYVA® plus LEUKERAN® and a combination of RITUXAN® plus LEUKERAN®. Five Hundred and eighty nine (589) treatment naïve CLL patients over 70 years of age with comorbidities were enrolled of whom 118 patients received LEUKERAN® alone, 238 received GAZYVA® plus LEUKERAN® and 233 received RITUXAN® plus LEUKERAN®. The primary endpoint was Progression-Free Survival (PFS). Chemoimmunotherapy with both GAZYVA® plus LEUKERAN® and RITUXAN® plus LEUKERAN® significantly prolonged PFS compared to LEUKERAN® alone. The median PFS was 11.1 months with LEUKERAN® alone compared to 26.7 months for GAZYVA® plus LEUKERAN® (HR=0.18, P<0.001) and 16.3 months for RITUXAN® plus LEUKERAN® (HR=0.44, P<0.001). This benefit was seen in all subgroups except those with del(17) and quality of life in those who received antibody along with LEUKERAN® was not compromised. The combination of GAZYVA® and LEUKERAN®, also prolonged overall survival when compared to LEUKERAN® alone (HR=0.41; P=0.002). This benefit however was not noted with the RITUXAN® plus LEUKERAN® combination. Treatment with GAZYVA® plus LEUKERAN® when compared with RITUXAN® plus LEUKERAN®, resulted in a longer PFS (26.7 vs15.2 months; HR=0.39; P<0.001), higher complete response rates (20.7% vs. 7.0%) and deeper molecular responses. Infusion related reactions were more common in the GAZYVA® plus LEUKERAN® group without increase in the risk for infections. The authors concluded that a combination of GAZYVA® and LEUKERAN® when given to elderly patients with comorbid conditions improved overall survival compared to LEUKERAN® alone and resulted in higher response rates and longer PFS than RITUXAN® plus LEUKERAN®. Goede V, Fischer K, Busch R, et al. N Engl J Med 2014; 370:1101-1110
Ceritinib in ALK-Rearranged Non–Small-Cell Lung Cancer
SUMMARY: EML4-ALK (Echinoderm Microtubule associated protein Like 4) – (Anaplastic Lymphoma Kinase) is an aberrant fusion-type oncoprotein and is a tyrosine kinase. This oncoprotein/tyrosine kinase is found in 2-7% of all Non Small Cell Lung Cancers (NSCLC) and is generated due to an inversion in the short arm of chromosome 2. This oncoprotein is more prevalent in patients with adenocarcinoma, who have little or no exposure to tobacco. Tyrosine kinases normally play an important role in cellular proliferation and differentiation. However with point mutations, translocation/rearrangement and amplification of the respective genes, the associated tyrosine kinases can potentially cause malignancy. Such is the case with mutations or translocations of the Anaplastic Lymphoma Kinase gene (ALK). XALKORI® (Crizotinib) is a small molecule Tyrosine Kinase Inhibitor that targets ALK, MET and ROS1 tyrosine kinases. In an open label phase III trial involving 347 patients with locally advanced or metastatic ALK-positive lung cancer who had received one prior platinum based regimen, treatment with XALKORI® significantly improved Progression Free Survival (PFS) and Response Rates (RR). In spite of this initial benefit, patients will however relapse within 12 months, with the average response duration of about 8 months. This has been attributed to acquired mutation within the ALK tyrosine kinase domain, amplification of the ALK fusion gene, subtherapeutic inhibition of ALK tyrosine kinase or activation of other pathways that can cause abnormal cell proliferation. Ceritinib (LDK378) is an oral, small molecule, second generation tyrosine kinase inhibitor of ALK and is 20 times as potent as XALKORI® against ALK. Unlike XALKORI®, Ceritinib does not inhibit MET kinase activity. Based on preclinical data supporting the efficacy of Ceritinib in both XALKORI® sensitive and resistant NSCLC tumors, the authors conducted a study to evaluate the antitumor activity of Ceritinib in patients with advanced NSCLC and other cancers harboring genetic alterations in ALK, in addition to determining the safety, MTD (maximum tolerated dose) and pharmacokinetics of Ceritinib. In this trial, patients who had received prior therapy with one or more ALK inhibitors as well as those with asymptomatic treated or untreated CNS metastases, were eligible to be enrolled. This study had 2 components – a dose escalation phase and an expansion phase. In the dose escalation phase, 59 patients were enrolled and the MTD of Ceritinib was determined to be 750 mg PO daily. In the expansion phase, 71 additional patients were treated for a total of 130 patients (N=59+71). Majority of these patients (94%) had advanced NSCLC. Patients with NSCLC who received at least 400mg of Ceritinib daily (N=114) had an overall response rate (RR) of 58% and median PFS was 7 months. Patients with advanced NSCLC who had received XALKORI® prior to enrollment (N=80) had a RR of 56%. The responses were noted both in patients with tumors harboring resistance mutations in the ALK tyrosine kinase domain as well as those in whom there was no new genetic alterations of ALK. Further, responses were seen in the untreated CNS lesions both in patients who had prior therapy with XALKORI® as well as those who did not. Adverse events were grade 1or 2 and GI related. These included vomiting, diarrhea, elevated aminotransferase levels and hypophosphatemia. The authors concluded that Cerifinib has marked antitumor activity in patients with advanced ALK rearranged NSCLC and in those who had progressed during XALKORI® treatment, regardless of the presence of resistance mutations in the ALK tyrosine kinase domain. Whether Cerifinib should be considered for the first line treatment of advanced ALK rearranged NSCLC, remains to be seen. Shaw AT, Kim D, Mehra R, et al. N Engl J Med 2014; 370:1189-1197
Newly Diagnosed Colorectal Cancer – NCCN Recommends Universal Screening for Lynch Syndrome
SUMMARY: Lynch Syndrome (Hereditary NonPolyposis Colorectal Cancer – HNPCC), is an autosomal dominant inherited disorder associated with increased risk of colorectal, endometrial, ovary, gastric, small bowel, pancreatic, brain, ureter or renal pelvis cancer. In the United States, approximately 140,000 new cases of colorectal cancer are diagnosed each year of which 3 to 5 percent are caused by Lynch Syndrome (LS). One in 35 patients with newly diagnosed colorectal cancer is related to Lynch Syndrome. Four genes, MLH1, MSH2, MSH6, and PMS2 are involved in the repair of mistakes that occur during DNA replication.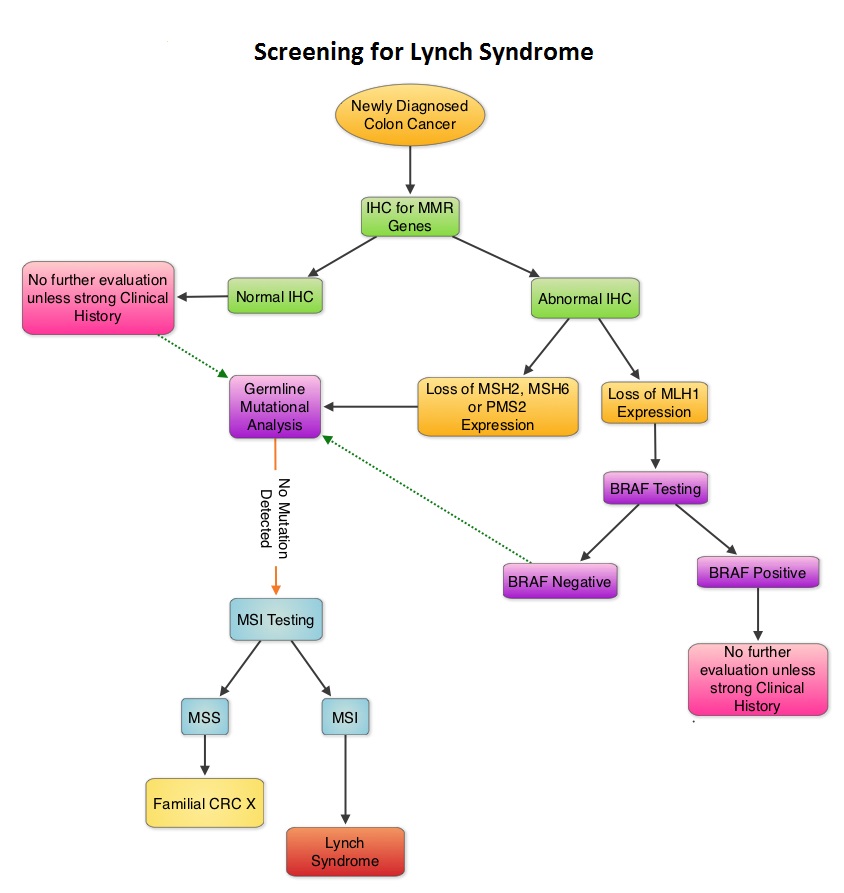 When any of these genes are mutated, repair of DNA replication mistakes is prevented resulting in continuous division of abnormal cells and possibly cancer. The EPCAM gene lies next to the MSH2 gene on chromosome 2 and mutations in the EPCAM gene can cause the MSH2 gene to be inactivated, interrupting DNA repair and leading to accumulation of DNA replication errors and possible malignancy. A Clinical Diagnosis of Lynch Syndrome can be made based on personal and family history if at least three relatives have a malignancy associated with Lynch Syndrome such as colorectal, endometrial, small bowel, ureter or renal pelvis cancer. In addition the following criteria should be met: • One relative must be a first-degree relative of the other two. • At least two successive generations must be affected. • At least one relative with a Lynch syndrome associated cancer should be diagnosed before 50 years of age. • Familial Adenomatous Polyposis should be excluded. • Tumors should be verified whenever possible. Because family history can sometimes be difficult to obtain or confirm NCCN in those circumstances has recommended screening all newly diagnosed colorectal cancer patients for Lynch syndrome. Germline defects/mutations in the mismatch repair genes MLH1, MSH2, MSH6 and PMS2 results in microsatellite instability in tumors. Tumors are described as MSI-High when they have changes in 2 or more, of the 5 microsatellite markers. So, High levels of MSI within a tumor are suggestive of defective DNA mismatch repair. ImmunoHistoChemistry (IHC) staining of tumor tissue is performed for protein expression of the four mismatch repair genes known to be mutated in Lynch Syndrome (MLH1, MSH2, MSH6 and PMS2). IHC test is described as normal when all 4 mismatch repair proteins are normally expressed suggesting that an underlying mismatch repair gene mutation is unlikely. When IHC test is abnormal, it means that that at least one of the 4 mismatch repair proteins is not expressed and an inherited mutation may be present in the gene related to that protein. This can be further confirmed by mutation analysis of the corresponding gene. Screening tests for Lynch syndrome include IHC staining of tumor tissue for protein expression of the four mismatch repair genes and tumor evaluation for MSI. In LS, more than 90% of the tumors are MSI-H (microsatellite instability-high) and/or lack expression of at least one of the mismatch repair proteins by IHC staining and there is a 96% correlation between IHC and MSI when used as a screening test for LS. Approximately 5% of tumors that display MSI may have normal protein expression for the four mismatch repair genes. It should be noted that an abnormal MSI and/or IHC test in colon cancer patients is not diagnostic of Lynch syndrome but can be a useful screening test. This is because even though MSI in the tumor tissue is pathognomonic of Lynch syndrome, approximately 15% of patients with sporadic colorectal cancers exhibit tumors with high MSI as a result of somatic MLH1 promoter hypermethylation. Further, the majority of colon cancer tumors that lack protein expression on IHC staining of MLH1 (often coexisting with loss of PMS2) are often due to an acquired genetic defect. If the IHC indicates absence of MLH1 protein expression, tumor should be tested for BRAF mutation V600E which can be seen in sporadic colorectal cancers but rarely found in patients who have Lynch Syndrome. Once a diagnosis of Lynch Syndrome is made, at risk family members should undergo colonoscopic evaluation at 20-25 years of age or 2-5 years prior to the earliest colon cancer, if it is diagnosed before age 25 and is repeated every 1-2 years. Prophylactic hysterectomy and bilateral salpingo-oophorectomy (BSO) should be considered by women who have completed childbearing. NCCN Guidelines Version 1.2014 Lynch Syndrome
When any of these genes are mutated, repair of DNA replication mistakes is prevented resulting in continuous division of abnormal cells and possibly cancer. The EPCAM gene lies next to the MSH2 gene on chromosome 2 and mutations in the EPCAM gene can cause the MSH2 gene to be inactivated, interrupting DNA repair and leading to accumulation of DNA replication errors and possible malignancy. A Clinical Diagnosis of Lynch Syndrome can be made based on personal and family history if at least three relatives have a malignancy associated with Lynch Syndrome such as colorectal, endometrial, small bowel, ureter or renal pelvis cancer. In addition the following criteria should be met: • One relative must be a first-degree relative of the other two. • At least two successive generations must be affected. • At least one relative with a Lynch syndrome associated cancer should be diagnosed before 50 years of age. • Familial Adenomatous Polyposis should be excluded. • Tumors should be verified whenever possible. Because family history can sometimes be difficult to obtain or confirm NCCN in those circumstances has recommended screening all newly diagnosed colorectal cancer patients for Lynch syndrome. Germline defects/mutations in the mismatch repair genes MLH1, MSH2, MSH6 and PMS2 results in microsatellite instability in tumors. Tumors are described as MSI-High when they have changes in 2 or more, of the 5 microsatellite markers. So, High levels of MSI within a tumor are suggestive of defective DNA mismatch repair. ImmunoHistoChemistry (IHC) staining of tumor tissue is performed for protein expression of the four mismatch repair genes known to be mutated in Lynch Syndrome (MLH1, MSH2, MSH6 and PMS2). IHC test is described as normal when all 4 mismatch repair proteins are normally expressed suggesting that an underlying mismatch repair gene mutation is unlikely. When IHC test is abnormal, it means that that at least one of the 4 mismatch repair proteins is not expressed and an inherited mutation may be present in the gene related to that protein. This can be further confirmed by mutation analysis of the corresponding gene. Screening tests for Lynch syndrome include IHC staining of tumor tissue for protein expression of the four mismatch repair genes and tumor evaluation for MSI. In LS, more than 90% of the tumors are MSI-H (microsatellite instability-high) and/or lack expression of at least one of the mismatch repair proteins by IHC staining and there is a 96% correlation between IHC and MSI when used as a screening test for LS. Approximately 5% of tumors that display MSI may have normal protein expression for the four mismatch repair genes. It should be noted that an abnormal MSI and/or IHC test in colon cancer patients is not diagnostic of Lynch syndrome but can be a useful screening test. This is because even though MSI in the tumor tissue is pathognomonic of Lynch syndrome, approximately 15% of patients with sporadic colorectal cancers exhibit tumors with high MSI as a result of somatic MLH1 promoter hypermethylation. Further, the majority of colon cancer tumors that lack protein expression on IHC staining of MLH1 (often coexisting with loss of PMS2) are often due to an acquired genetic defect. If the IHC indicates absence of MLH1 protein expression, tumor should be tested for BRAF mutation V600E which can be seen in sporadic colorectal cancers but rarely found in patients who have Lynch Syndrome. Once a diagnosis of Lynch Syndrome is made, at risk family members should undergo colonoscopic evaluation at 20-25 years of age or 2-5 years prior to the earliest colon cancer, if it is diagnosed before age 25 and is repeated every 1-2 years. Prophylactic hysterectomy and bilateral salpingo-oophorectomy (BSO) should be considered by women who have completed childbearing. NCCN Guidelines Version 1.2014 Lynch Syndrome
