SUMMARY: The CDC estimates that approximately 1-2 per 1000 individuals develop Deep Vein Thrombosis/Pulmonary Embolism (PE) each year in the United States, resulting in 60,000-100,000 deaths. Even though Deep Vein Thrombosis (DVT) commonly occurs in the lower extremities, Non-Leg Deep Venous Thromboses (NLDVT) at other sites including the head and neck, trunk, and upper extremities can occur. The incidence, risk factors, management and outcomes in this patient group remains unclear. The authors therefore conducted a prospective cohort study in the ICU setting. This study is nested in a larger international trial in which 3746 patients, expected to remain in the med/surg Intensive Care Unit (ICU) for at least 3 days, were randomized to receive either Unfractionated (standard) heparin or Dalteparin (FRAGMIN®) for thromboprophylaxis.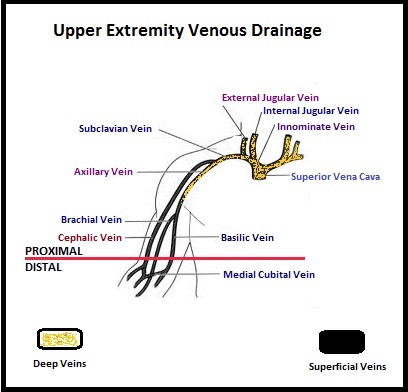 The authors characterized the NLDVT as Prevalent or Incident (depending on whether the thrombosis was identified within 72 hours of ICU admission or developed after the third ICU day) and whether they were catheter related or not. Risk factors for NLDVT and subsequent anticoagulant therapy, associated PE, and death were evaluated. Several important findings were noted from this study. Of the 3746 patients, 2.2% developed 1 or more Non-Leg Vein Thromboses (superficial or deep, proximal or distal). Majority of these thrombotic events (95%) occurred in the upper extremity and most of these occurred in the clinically important proximal and deep venous system. Further, these thrombotic episodes were more commonly Incident (2.0%) rather than Prevalent (0.2%) – P <0.001. It was noted that 1 in 7 patients with NLDVT developed PE. It appears that malignancy, hospitalization and Central Venous Catheters are risk factors for Upper Extremity Deep Vein Thrombosis (UEDVT), but the disproportionate increase in the incidence of UEDVT in hospitalized patients (30%-40% of hospital associated DVT’s) has been attributed to the increased use of CVC’s such as PICC (Peripherally Inserted Central Catheter). In this study, only 13% of the patients diagnosed with NLDVT received anticoagulation therapy. In an accompanying commentary by Dr. Greg Maynard, it was well pointed out that UEDVT is associated with similar rates of recurrence, PE and mortality as lower extremity DVT and as such UEDVT should be treated with anticoagulant therapy similar to Lower Extremity DVT. Dr. Maynard also noted that PICC associated DVT could be significantly reduced by appropriately choosing patients for a PICC line, proper PICC placement, early PICC removal, smaller diameter PICC and smaller number of lumens in the catheter. The authors concluded that despite universal prophylaxis with heparin, there was a high incidence of NLDVT in clinically important locations of the venous system, in critically ill patients and this calls for more structured preventive measures, as we learn more about this entity. Lamontagne F, McIntyre L, Dodek P, et al. JAMA Intern Med. 2014;174:689-696
The authors characterized the NLDVT as Prevalent or Incident (depending on whether the thrombosis was identified within 72 hours of ICU admission or developed after the third ICU day) and whether they were catheter related or not. Risk factors for NLDVT and subsequent anticoagulant therapy, associated PE, and death were evaluated. Several important findings were noted from this study. Of the 3746 patients, 2.2% developed 1 or more Non-Leg Vein Thromboses (superficial or deep, proximal or distal). Majority of these thrombotic events (95%) occurred in the upper extremity and most of these occurred in the clinically important proximal and deep venous system. Further, these thrombotic episodes were more commonly Incident (2.0%) rather than Prevalent (0.2%) – P <0.001. It was noted that 1 in 7 patients with NLDVT developed PE. It appears that malignancy, hospitalization and Central Venous Catheters are risk factors for Upper Extremity Deep Vein Thrombosis (UEDVT), but the disproportionate increase in the incidence of UEDVT in hospitalized patients (30%-40% of hospital associated DVT’s) has been attributed to the increased use of CVC’s such as PICC (Peripherally Inserted Central Catheter). In this study, only 13% of the patients diagnosed with NLDVT received anticoagulation therapy. In an accompanying commentary by Dr. Greg Maynard, it was well pointed out that UEDVT is associated with similar rates of recurrence, PE and mortality as lower extremity DVT and as such UEDVT should be treated with anticoagulant therapy similar to Lower Extremity DVT. Dr. Maynard also noted that PICC associated DVT could be significantly reduced by appropriately choosing patients for a PICC line, proper PICC placement, early PICC removal, smaller diameter PICC and smaller number of lumens in the catheter. The authors concluded that despite universal prophylaxis with heparin, there was a high incidence of NLDVT in clinically important locations of the venous system, in critically ill patients and this calls for more structured preventive measures, as we learn more about this entity. Lamontagne F, McIntyre L, Dodek P, et al. JAMA Intern Med. 2014;174:689-696
Author: RR
Bevacizumab Combined With Chemotherapy for Platinum-Resistant Recurrent Ovarian Cancer The AURELIA Open-Label Randomized Phase III Trial
SUMMARY: It is estimated that in the United States, approximately 22,000 women will be diagnosed with ovarian cancer in 2014 and a little over 14,000 women will die of the disease. In spite of significantly improved median survival following aggressive surgical debulking and platinum plus taxane based therapy, long term cure rate is approximately 20-30%. Majority of the patients relapse in 18-24 months and 25% of these patients are Platinum Resistant. These platinum resistant patients are usually treated with single agent chemotherapy drugs such as DOXIL® (Pegylated Liposomal Doxorubicin-PLD), TAXOL® (Paclitaxel) and HYCAMTIN® (Topotecan), with an expected response rate of 10-15%, median response duration of about 3-4 months and median Overall Survival of approximately 12 months.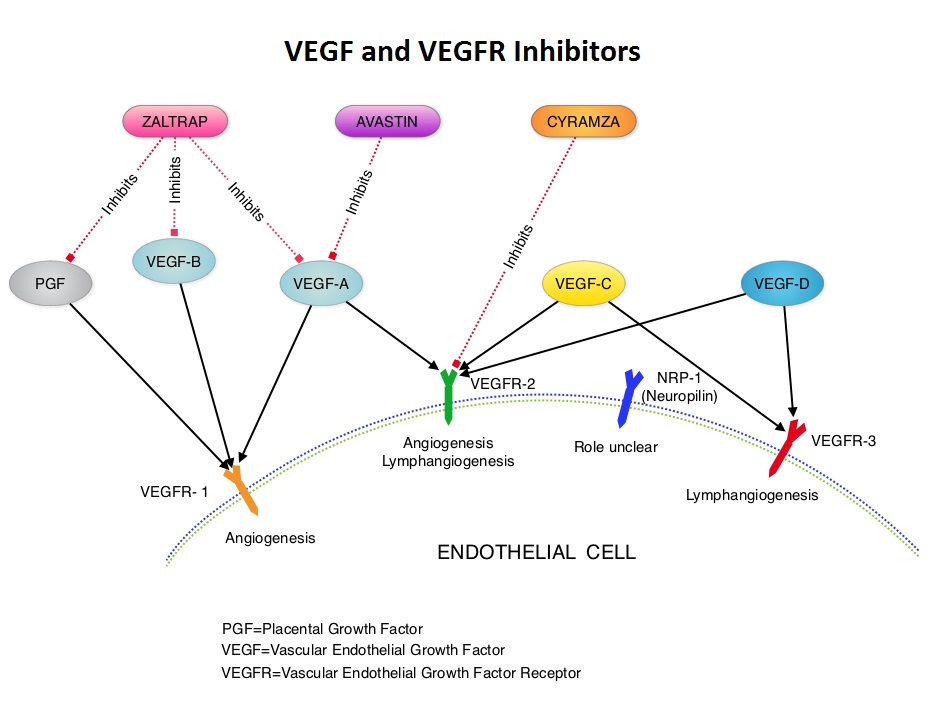 AURELIA (Avastin Use in Platinum-Resistant Epithelial Ovarian Cancer) is a multicenter, randomized, open-label, Phase III study in which 361 women with platinum resistant recurrent epithelial ovarian, primary peritoneal or fallopian tube cancer were enrolled. These patients had disease progression within six months of their platinum based chemotherapy (Platinum Resistant) and were randomly assigned to receive AVASTIN® (Bevacizumab) 10 mg/kg every 2 weeks or 15 mg/kg every 3 weeks in combination with investigators choice of single agent chemotherapy agent such as weekly TAXOL®, HYCAMTIN®, DOXIL® (N=179) or single agent chemotherapy alone (N=182). Patients with refractory disease, history of bowel obstruction, or those who had received two or more prior anticancer regimens were excluded. Treatment was given until disease progression. Patients who had progressed on single agent chemotherapy were allowed to cross over to single agent AVASTIN®. The primary end point was Progression Free Survival (PFS) and secondary end points included Objective Response Rate (ORR), Overall Survival (OS), safety, and patient reported outcomes. The combination of AVASTIN® plus chemotherapy resulted in a 52% reduction in the risk of progression compared to those who received chemotherapy alone, with a median PFS of 6.7 months for the AVASTIN® plus chemotherapy group vs 3.4 months for the single agent chemotherapy group (HR=0.48, P<0.001) and thus met the primary endpoint of this clinical trial. This PFS benefit was seen consistently across all subgroups including the subgroup of patients with ascites. The ORR was 27.3% with the AVASTIN® combination vs 11.8% with single agent chemotherapy (P =0.001). The median OS was 16.6 months for the AVASTIN® combination vs 13.3 months for the single agent chemotherapy group (HR=0.85; P < .17). The lack of statistical significance in the OS has been attributed to cross over of 40% of patients, initially randomized to the chemotherapy alone group, who upon progression, received single agent AVASTIN®. As expected, grade 2 or more hypertension and proteinuria were common in the AVASTIN® group and GI perforation occurred in 2.2% of these patients. There was a 15% improvement in abdominal and GI symptoms as reported by patients, with the AVASTIN® combination, compared to chemotherapy alone. The authors concluded that AVASTIN® in combination with chemotherapy significantly improved Progression Free Survival and Objective Response Rates in patients with Platinum Resistant recurrent Ovarian Cancer. Pujade-Lauraine E, Hilpert F, Weber B, et al. J Clin Oncol 2014;32:1302-1308
AURELIA (Avastin Use in Platinum-Resistant Epithelial Ovarian Cancer) is a multicenter, randomized, open-label, Phase III study in which 361 women with platinum resistant recurrent epithelial ovarian, primary peritoneal or fallopian tube cancer were enrolled. These patients had disease progression within six months of their platinum based chemotherapy (Platinum Resistant) and were randomly assigned to receive AVASTIN® (Bevacizumab) 10 mg/kg every 2 weeks or 15 mg/kg every 3 weeks in combination with investigators choice of single agent chemotherapy agent such as weekly TAXOL®, HYCAMTIN®, DOXIL® (N=179) or single agent chemotherapy alone (N=182). Patients with refractory disease, history of bowel obstruction, or those who had received two or more prior anticancer regimens were excluded. Treatment was given until disease progression. Patients who had progressed on single agent chemotherapy were allowed to cross over to single agent AVASTIN®. The primary end point was Progression Free Survival (PFS) and secondary end points included Objective Response Rate (ORR), Overall Survival (OS), safety, and patient reported outcomes. The combination of AVASTIN® plus chemotherapy resulted in a 52% reduction in the risk of progression compared to those who received chemotherapy alone, with a median PFS of 6.7 months for the AVASTIN® plus chemotherapy group vs 3.4 months for the single agent chemotherapy group (HR=0.48, P<0.001) and thus met the primary endpoint of this clinical trial. This PFS benefit was seen consistently across all subgroups including the subgroup of patients with ascites. The ORR was 27.3% with the AVASTIN® combination vs 11.8% with single agent chemotherapy (P =0.001). The median OS was 16.6 months for the AVASTIN® combination vs 13.3 months for the single agent chemotherapy group (HR=0.85; P < .17). The lack of statistical significance in the OS has been attributed to cross over of 40% of patients, initially randomized to the chemotherapy alone group, who upon progression, received single agent AVASTIN®. As expected, grade 2 or more hypertension and proteinuria were common in the AVASTIN® group and GI perforation occurred in 2.2% of these patients. There was a 15% improvement in abdominal and GI symptoms as reported by patients, with the AVASTIN® combination, compared to chemotherapy alone. The authors concluded that AVASTIN® in combination with chemotherapy significantly improved Progression Free Survival and Objective Response Rates in patients with Platinum Resistant recurrent Ovarian Cancer. Pujade-Lauraine E, Hilpert F, Weber B, et al. J Clin Oncol 2014;32:1302-1308
AFINITOR® overcomes Endocrine Resistance in Breast Cancer
AFINITOR® can overcome endocrine resistance in patients with Metastatic Breast Cancer. This was demonstrated in the BOLERO-2 trial in which patients who had progressed on non-steroidal Aromatase Inhibitors, when treated with a combination of steroidal Aromatase Inhibitor AROMASIN® (Exemestane) and AFINITOR® (Everolimus), had significantly improved Progression Free Survival and Clinical Benefit. AFINITOR® is a mTOR inhibitor and mTOR pathway has been implicated as one of the mechanisms for endocrine resistance in Breast cancer. A recent BOLERO-2 trial update, was published in the Breast Cancer Research and Treatment 2013.
Analysis of KRAS/NRAS mutations in phase 3 study 20050181 of panitumumab (pmab) plus FOLFIRI versus FOLFIRI for second-line treatment (tx) of metastatic colorectal cancer (mCRC)
SUMMARY: It is estimated that approximately 97,000 new cases of colon cancer will be diagnosed in 2014 and over 50,000 will die of the disease. The lifetime risk of developing colorectal cancer is about 1 in 20. Even though colon cancer localized to the bowel is potentially curable with surgery and adjuvant chemotherapy, advanced colon cancer is often incurable. Multi agent chemotherapy in combination with monoclonal antibodies, targeted against Epidermal Growth Factor Receptor (EGFR) such as VECTIBIX® (Panitumumab) and ERBITUX® (Cetuximab), have demonstrated survival benefit, for patients with metastatic colon cancer, whose tumors do not harbor KRAS mutations in exon 2. The authors in this study evaluated the benefit of testing metastatic colorectal cancer tumors for RAS mutations beyond the mutations in the KRAS gene at exon 2.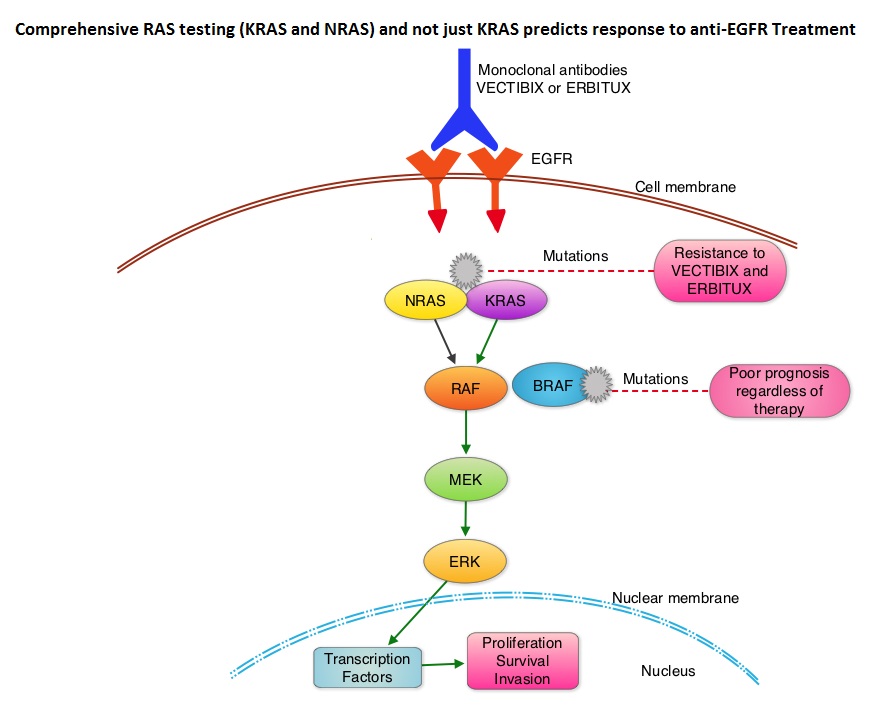 The most common RAS oncogenes in human cancer are HRAS, KRAS, and NRAS. Mutations in HRAS are not common in colon cancer whereas KRAS and NRAS mutations are seen in colon cancer and tend to be mutually exclusive. Activating mutations in exon 2 of the KRAS gene is seen in about 40% of colon cancer patients and predicts resistance to EGFR therapy. Mutational analysis is therefore usually performed to look for mutations in exon 2 of the KRAS gene. It appears however that a broader assessment of the RAS genes may more accurately predict resistance to EGFR therapy. It is also known that mutations in BRAF gene, which is downstream from RAS, may confer poor prognosis in colon cancer, regardless of therapy. The authors in a previous publication showed that in a phase III study involving 1,186 patients, the addition of VECTIBIX®, a fully human, EGFR targeted, monoclonal antibody, when combined with FOLFIRI (Folinic acid, Fluorouracil and Irinotecan), significantly improved Progression Free Survival, when compared to FOLFIRI alone (HR=0.73; P=0.004), with a trend towards improved Overall Survival (HR=0.85; P=0.12). Evolving data has suggested that testing for additional mutations in the RAS genes may help better understand the efficacy of/resistance to VECTIBIX®. In this present analysis, tumor samples of patients from the authors previous study, that were already known to be Wild Type KRAS – unmutated at KRAS exon 2 (N=597), were assessed for additional RAS mutations, specifically in KRAS exons 3 and 4 and NRAS exons 2, 3 and 4. Eighteen percent (18%) of the Wild Type KRAS exon 2 patients harbored additional RAS mutations. It was noted that patients receiving VECTIBIX® along with FOLFIRI had an improvement in both the median OS and PFS when they had wild-type (unmutated) RAS tumors compared to those, whose tumors harbored RAS mutations (median OS: 16.2 vs 11.8 months; median PFS: 6.4 vs 4.8 months). More importantly, amongst patients with mutated RAS tumors, the addition of VECTIBIX® to FOLFIRI resulted in no significant survival benefit compared to FOLFIRI alone (median OS: 11.8 vs 11.1 months; median PFS: 4.8 vs 4.0 months). The authors concluded that comprehensive RAS mutational analysis rather than KRAS testing alone, gives more relevant information for proper selection of patients with metastatic colorectal cancer, who would benefit from EGFR targeted monoclonal antibodies such as VECTIBIX®. In addition patients with RAS mutations could be spared from the associated cost and toxicites of EGFR targeted monoclonal antibodies that will not improve their outcomes. Peeters M, Oliner KS, Price TJ, et al. J Clin Oncol 32, 2014 (suppl 3; abstr LBA387)
The most common RAS oncogenes in human cancer are HRAS, KRAS, and NRAS. Mutations in HRAS are not common in colon cancer whereas KRAS and NRAS mutations are seen in colon cancer and tend to be mutually exclusive. Activating mutations in exon 2 of the KRAS gene is seen in about 40% of colon cancer patients and predicts resistance to EGFR therapy. Mutational analysis is therefore usually performed to look for mutations in exon 2 of the KRAS gene. It appears however that a broader assessment of the RAS genes may more accurately predict resistance to EGFR therapy. It is also known that mutations in BRAF gene, which is downstream from RAS, may confer poor prognosis in colon cancer, regardless of therapy. The authors in a previous publication showed that in a phase III study involving 1,186 patients, the addition of VECTIBIX®, a fully human, EGFR targeted, monoclonal antibody, when combined with FOLFIRI (Folinic acid, Fluorouracil and Irinotecan), significantly improved Progression Free Survival, when compared to FOLFIRI alone (HR=0.73; P=0.004), with a trend towards improved Overall Survival (HR=0.85; P=0.12). Evolving data has suggested that testing for additional mutations in the RAS genes may help better understand the efficacy of/resistance to VECTIBIX®. In this present analysis, tumor samples of patients from the authors previous study, that were already known to be Wild Type KRAS – unmutated at KRAS exon 2 (N=597), were assessed for additional RAS mutations, specifically in KRAS exons 3 and 4 and NRAS exons 2, 3 and 4. Eighteen percent (18%) of the Wild Type KRAS exon 2 patients harbored additional RAS mutations. It was noted that patients receiving VECTIBIX® along with FOLFIRI had an improvement in both the median OS and PFS when they had wild-type (unmutated) RAS tumors compared to those, whose tumors harbored RAS mutations (median OS: 16.2 vs 11.8 months; median PFS: 6.4 vs 4.8 months). More importantly, amongst patients with mutated RAS tumors, the addition of VECTIBIX® to FOLFIRI resulted in no significant survival benefit compared to FOLFIRI alone (median OS: 11.8 vs 11.1 months; median PFS: 4.8 vs 4.0 months). The authors concluded that comprehensive RAS mutational analysis rather than KRAS testing alone, gives more relevant information for proper selection of patients with metastatic colorectal cancer, who would benefit from EGFR targeted monoclonal antibodies such as VECTIBIX®. In addition patients with RAS mutations could be spared from the associated cost and toxicites of EGFR targeted monoclonal antibodies that will not improve their outcomes. Peeters M, Oliner KS, Price TJ, et al. J Clin Oncol 32, 2014 (suppl 3; abstr LBA387)
Everolimus plus exemestane as first-line therapy in HR+, HER2− advanced breast cancer in BOLERO-2
SUMMARY:Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately, 233,000 new cases of invasive breast cancer will be diagnosed in 2014 and 40,000 women will die of the disease. Approximately 75% of patients with breast cancer are hormone receptor positive and 15%-20% of breast cancers overexpress HER2/neu oncogene. As such a significant number of breast cancers are Hormone Receptor (HR) positive and HER2 negative. Aromatase Inhibitors (AI’s) are often prescribed, due to their superiority over Tamoxifen, for post menopausal women with HR+ and HER2-negative breast tumors, both in adjuvant as well as metastatic settings. These patients will eventually develop progressive disease on endocrine therapy with AI’s, attributed to endocrine resistance. The average Progression Free Survival for these patients is 4-6 months when treated with other hormonal interventions including higher doses of FASLODEX® (Fulvestrant). Further, FASLODEX® has not been shown to be superior to steroidal or non steroidal AI’s.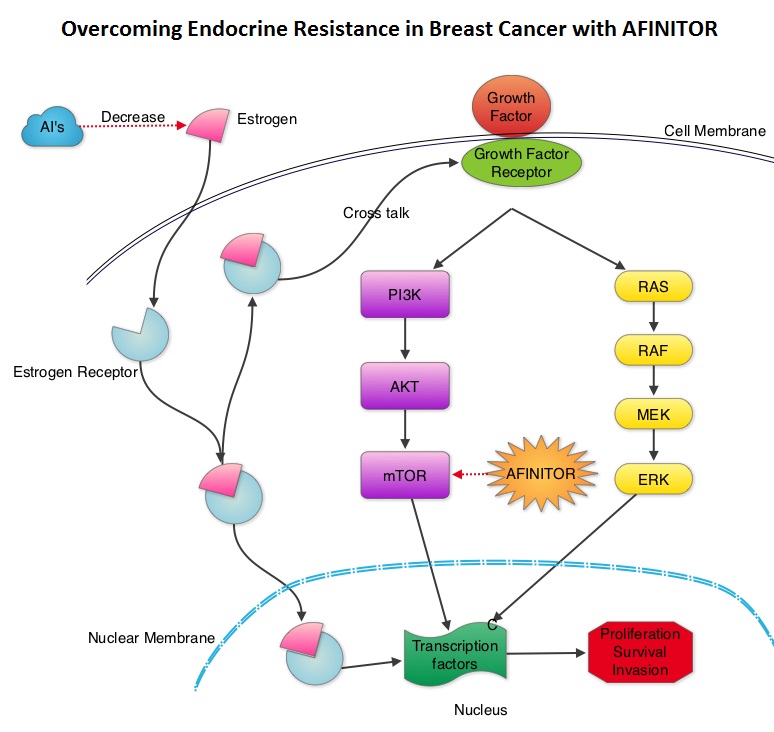 The mechanism of endocrine resistance has been attributed to cross talk between Estrogen Receptor signaling and PI3K/AKT/mTOR pathway. The PI3K/AKT/mTOR is a complex pathway, essential for cell proliferation, survival and apoptosis (programmed cell death). This pathway is of interest because of its increased activity in malignant cells as a result of amplification or mutation of the genes associated with PI3-kinase (phosphatidylinositol-3 kinase) and AKT, as well as loss of function of PTEN. PTEN normally prevents activation of AKT and its downstream pathways. Therefore, elevated ER signaling and breast cancer progression has been associated with activation of this mTOR (mammalian Target Of Rapamycin) pathway. Everolimus (AFINITOR®) is a mTOR inhibitor that has been shown to inhibit ER signaling and restore sensitivity to anti-estrogen therapies. With this preclinical knowledge, the Breast cancer trial of OraL EveROlimus-2 (BOLERO-2), which is a randomized, multicenter phase III trial was conducted to evaluate the benefit of combining steroidal AI, AROMASIN® (Exemestane) and an mTOR inhibitor AFINITOR®, for treatment of postmenopausal patients with HR+ and HER2 negative advanced breast cancer, who had either recurrent or progressive disease after non steroidal AI’s. Seven hundred and twenty four (N=724) patients were randomly assigned in a 2:1 ratio to receive either a combination of a steroidal AI, AROMASIN® at 25 mg PO QD and AFINITOR® at 10 mg PO QD (N=485) or AROMASIN® along with a placebo (N=239). Both treatment groups were well balanced and patients were stratified according to sensitivity to previous hormonal therapy and presence of visceral metastases. The primary endpoint for this study was Progression Free Survival (PFS) and secondary endpoints included overall Response Rate (RR), Clinical Benefit Rate (CBR defined as complete response + partial response + stable disease for at least 6 months), Overall Survival, Quality of Life, changes in bone marker levels and patient safety. In this study, close to 60% of the patients had visceral disease and approximately 80% of the patients had prior therapies for metastatic disease, with only 20% receiving the study drugs as their first therapy for metastatic disease. The combination of AFINITOR® and AROMASIN® significantly prolonged PFS compared to AROMASIN® alone (11 vs 4.1 months, HR=0.38, P<0.0001) and the Clinical Benefit Rate was 51% in the combination group and 26% in the AROMASIN® alone group (P<0.0001). The combination of AFINITOR® and AROMASIN® benefitted all subgroups of patients including those who had disease recurrence during or after neoadjuvant/adjuvant non steroidal AI therapy, those with visceral and bone metastases, as well as those who had prior chemotherapy. The most common adverse events in all age groups were rash, stomatitis, fatigue, diarrhea and nausea and these toxicities were manageable. The authors concluded that AFINITOR® given along with AROMASIN® in the study population can decrease the risk of disease progression by 60% and can double the Clinical Benefit Rate, compared to AROMASIN® alone, even in patients 65 years of age or older, thereby delaying the need for chemotherapy. Beck JT, Hortobagyi GN, Campone M, et al. Breast Cancer Res Treat. 2014; 143: 459–467
The mechanism of endocrine resistance has been attributed to cross talk between Estrogen Receptor signaling and PI3K/AKT/mTOR pathway. The PI3K/AKT/mTOR is a complex pathway, essential for cell proliferation, survival and apoptosis (programmed cell death). This pathway is of interest because of its increased activity in malignant cells as a result of amplification or mutation of the genes associated with PI3-kinase (phosphatidylinositol-3 kinase) and AKT, as well as loss of function of PTEN. PTEN normally prevents activation of AKT and its downstream pathways. Therefore, elevated ER signaling and breast cancer progression has been associated with activation of this mTOR (mammalian Target Of Rapamycin) pathway. Everolimus (AFINITOR®) is a mTOR inhibitor that has been shown to inhibit ER signaling and restore sensitivity to anti-estrogen therapies. With this preclinical knowledge, the Breast cancer trial of OraL EveROlimus-2 (BOLERO-2), which is a randomized, multicenter phase III trial was conducted to evaluate the benefit of combining steroidal AI, AROMASIN® (Exemestane) and an mTOR inhibitor AFINITOR®, for treatment of postmenopausal patients with HR+ and HER2 negative advanced breast cancer, who had either recurrent or progressive disease after non steroidal AI’s. Seven hundred and twenty four (N=724) patients were randomly assigned in a 2:1 ratio to receive either a combination of a steroidal AI, AROMASIN® at 25 mg PO QD and AFINITOR® at 10 mg PO QD (N=485) or AROMASIN® along with a placebo (N=239). Both treatment groups were well balanced and patients were stratified according to sensitivity to previous hormonal therapy and presence of visceral metastases. The primary endpoint for this study was Progression Free Survival (PFS) and secondary endpoints included overall Response Rate (RR), Clinical Benefit Rate (CBR defined as complete response + partial response + stable disease for at least 6 months), Overall Survival, Quality of Life, changes in bone marker levels and patient safety. In this study, close to 60% of the patients had visceral disease and approximately 80% of the patients had prior therapies for metastatic disease, with only 20% receiving the study drugs as their first therapy for metastatic disease. The combination of AFINITOR® and AROMASIN® significantly prolonged PFS compared to AROMASIN® alone (11 vs 4.1 months, HR=0.38, P<0.0001) and the Clinical Benefit Rate was 51% in the combination group and 26% in the AROMASIN® alone group (P<0.0001). The combination of AFINITOR® and AROMASIN® benefitted all subgroups of patients including those who had disease recurrence during or after neoadjuvant/adjuvant non steroidal AI therapy, those with visceral and bone metastases, as well as those who had prior chemotherapy. The most common adverse events in all age groups were rash, stomatitis, fatigue, diarrhea and nausea and these toxicities were manageable. The authors concluded that AFINITOR® given along with AROMASIN® in the study population can decrease the risk of disease progression by 60% and can double the Clinical Benefit Rate, compared to AROMASIN® alone, even in patients 65 years of age or older, thereby delaying the need for chemotherapy. Beck JT, Hortobagyi GN, Campone M, et al. Breast Cancer Res Treat. 2014; 143: 459–467
ZYKADIA® (Ceritinib)
The FDA on April 29, 2014 granted accelerated approval to ZYKADIA® for the treatment of patients with Anaplastic Lymphoma Kinase (ALK)-positive, metastatic Non-Small Cell Lung Cancer (NSCLC), with disease progression on or who are intolerant to XALKORI® (Crizotinib). ZYKADIA® capsules are a product of Novartis Pharmaceuticals Corporation.
PURIXAN® (Mercaptopurine)
The FDA on April 28, 2014 approved PURIXAN® for the treatment of patients with Acute Lymphoblastic Leukemia (ALL) as part of a combination regimen. PURIXAN® oral suspension is a product of NOVA Laboratories Limited.
SYLVANT® (Siltuximab)
The FDA on April 23, 2014 approved SYLVANT® for the treatment of patients with multicentric Castleman’s disease (MCD) who are human immunodeficiency virus (HIV-) -negative and human herpes virus -8 (HHV-8) -negative. SYLVANT® injection is a product of Janssen Biotech, Inc.
CYRAMZA® (Ramucirumab)
The FDA on April 21, 2014 approved CYRAMZA® for use as a single agent, for the treatment of patients with advanced or metastatic, gastric or gastroesophageal junction (GEJ) adenocarcinoma, with disease progression on or after prior treatment with fluoropyrimidine- or platinum-containing chemotherapy. CYRAMZA® injection for intravenous infusion is a product of Eli Lilly and Company.
Yoga Can Improve Sense of Well Being in Breast Cancer Survivors
It appears that Yoga can substantially reduce Inflammation, Fatigue and improve Vitality in breast cancer survivors. This was substantiated in a randomized trial, which enrolled breast cancer patients following local intervention and adjuvant chemotherapy. The authors were also able to measure and demonstrate a drop in the cytokines associated with inflammation, such as Interleukin-6 (IL-6), Interleukin-1beta (IL-1b) and Tumor Necrosis Factor-alfa (TNFa), with Yoga intervention. These interesting and intriguing findings were published in the Journal of Clinical Oncology. A summary of this study is available to review at www.oncoprescribe.com.
