SUMMARY:Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately, 233,000 new cases of invasive breast cancer will be diagnosed in 2014 and 40,000 women will die of the disease. Approximately 75% of patients with breast cancer are hormone receptor positive and 15%-20% of breast cancers overexpress HER2/neu oncogene. As such a significant number of breast cancers are Hormone Receptor (HR) positive and HER2 negative. Aromatase Inhibitors (AI’s) are often prescribed, due to their superiority over Tamoxifen, for post menopausal women with HR+ and HER2-negative breast tumors, both in adjuvant as well as metastatic settings. These patients will eventually develop progressive disease on endocrine therapy with AI’s, attributed to endocrine resistance. The average Progression Free Survival for these patients is 4-6 months when treated with other hormonal interventions including higher doses of FASLODEX® (Fulvestrant). Further, FASLODEX® has not been shown to be superior to steroidal or non steroidal AI’s.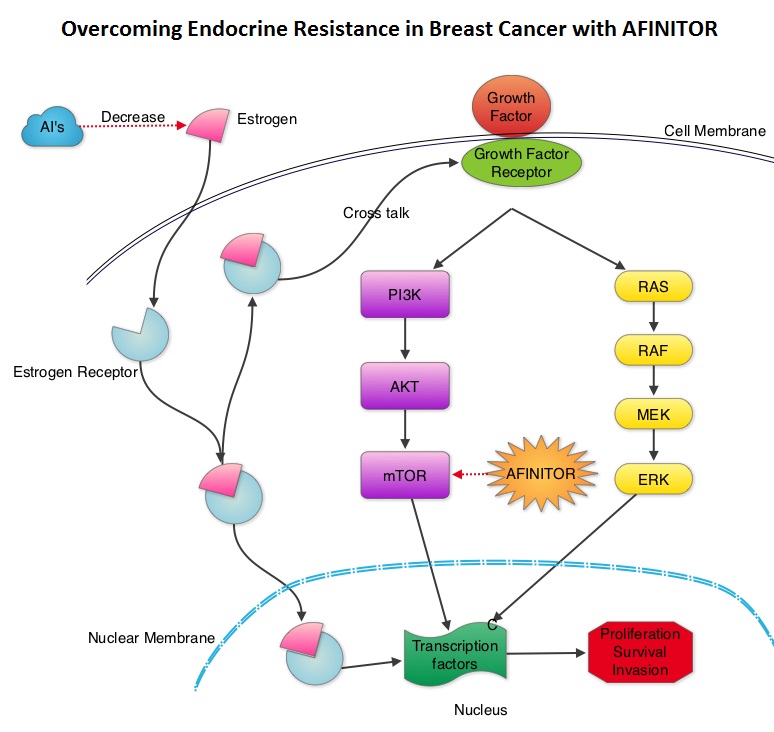 The mechanism of endocrine resistance has been attributed to cross talk between Estrogen Receptor signaling and PI3K/AKT/mTOR pathway. The PI3K/AKT/mTOR is a complex pathway, essential for cell proliferation, survival and apoptosis (programmed cell death). This pathway is of interest because of its increased activity in malignant cells as a result of amplification or mutation of the genes associated with PI3-kinase (phosphatidylinositol-3 kinase) and AKT, as well as loss of function of PTEN. PTEN normally prevents activation of AKT and its downstream pathways. Therefore, elevated ER signaling and breast cancer progression has been associated with activation of this mTOR (mammalian Target Of Rapamycin) pathway. Everolimus (AFINITOR®) is a mTOR inhibitor that has been shown to inhibit ER signaling and restore sensitivity to anti-estrogen therapies. With this preclinical knowledge, the Breast cancer trial of OraL EveROlimus-2 (BOLERO-2), which is a randomized, multicenter phase III trial was conducted to evaluate the benefit of combining steroidal AI, AROMASIN® (Exemestane) and an mTOR inhibitor AFINITOR®, for treatment of postmenopausal patients with HR+ and HER2 negative advanced breast cancer, who had either recurrent or progressive disease after non steroidal AI’s. Seven hundred and twenty four (N=724) patients were randomly assigned in a 2:1 ratio to receive either a combination of a steroidal AI, AROMASIN® at 25 mg PO QD and AFINITOR® at 10 mg PO QD (N=485) or AROMASIN® along with a placebo (N=239). Both treatment groups were well balanced and patients were stratified according to sensitivity to previous hormonal therapy and presence of visceral metastases. The primary endpoint for this study was Progression Free Survival (PFS) and secondary endpoints included overall Response Rate (RR), Clinical Benefit Rate (CBR defined as complete response + partial response + stable disease for at least 6 months), Overall Survival, Quality of Life, changes in bone marker levels and patient safety. In this study, close to 60% of the patients had visceral disease and approximately 80% of the patients had prior therapies for metastatic disease, with only 20% receiving the study drugs as their first therapy for metastatic disease. The combination of AFINITOR® and AROMASIN® significantly prolonged PFS compared to AROMASIN® alone (11 vs 4.1 months, HR=0.38, P<0.0001) and the Clinical Benefit Rate was 51% in the combination group and 26% in the AROMASIN® alone group (P<0.0001). The combination of AFINITOR® and AROMASIN® benefitted all subgroups of patients including those who had disease recurrence during or after neoadjuvant/adjuvant non steroidal AI therapy, those with visceral and bone metastases, as well as those who had prior chemotherapy. The most common adverse events in all age groups were rash, stomatitis, fatigue, diarrhea and nausea and these toxicities were manageable. The authors concluded that AFINITOR® given along with AROMASIN® in the study population can decrease the risk of disease progression by 60% and can double the Clinical Benefit Rate, compared to AROMASIN® alone, even in patients 65 years of age or older, thereby delaying the need for chemotherapy. Beck JT, Hortobagyi GN, Campone M, et al. Breast Cancer Res Treat. 2014; 143: 459–467
The mechanism of endocrine resistance has been attributed to cross talk between Estrogen Receptor signaling and PI3K/AKT/mTOR pathway. The PI3K/AKT/mTOR is a complex pathway, essential for cell proliferation, survival and apoptosis (programmed cell death). This pathway is of interest because of its increased activity in malignant cells as a result of amplification or mutation of the genes associated with PI3-kinase (phosphatidylinositol-3 kinase) and AKT, as well as loss of function of PTEN. PTEN normally prevents activation of AKT and its downstream pathways. Therefore, elevated ER signaling and breast cancer progression has been associated with activation of this mTOR (mammalian Target Of Rapamycin) pathway. Everolimus (AFINITOR®) is a mTOR inhibitor that has been shown to inhibit ER signaling and restore sensitivity to anti-estrogen therapies. With this preclinical knowledge, the Breast cancer trial of OraL EveROlimus-2 (BOLERO-2), which is a randomized, multicenter phase III trial was conducted to evaluate the benefit of combining steroidal AI, AROMASIN® (Exemestane) and an mTOR inhibitor AFINITOR®, for treatment of postmenopausal patients with HR+ and HER2 negative advanced breast cancer, who had either recurrent or progressive disease after non steroidal AI’s. Seven hundred and twenty four (N=724) patients were randomly assigned in a 2:1 ratio to receive either a combination of a steroidal AI, AROMASIN® at 25 mg PO QD and AFINITOR® at 10 mg PO QD (N=485) or AROMASIN® along with a placebo (N=239). Both treatment groups were well balanced and patients were stratified according to sensitivity to previous hormonal therapy and presence of visceral metastases. The primary endpoint for this study was Progression Free Survival (PFS) and secondary endpoints included overall Response Rate (RR), Clinical Benefit Rate (CBR defined as complete response + partial response + stable disease for at least 6 months), Overall Survival, Quality of Life, changes in bone marker levels and patient safety. In this study, close to 60% of the patients had visceral disease and approximately 80% of the patients had prior therapies for metastatic disease, with only 20% receiving the study drugs as their first therapy for metastatic disease. The combination of AFINITOR® and AROMASIN® significantly prolonged PFS compared to AROMASIN® alone (11 vs 4.1 months, HR=0.38, P<0.0001) and the Clinical Benefit Rate was 51% in the combination group and 26% in the AROMASIN® alone group (P<0.0001). The combination of AFINITOR® and AROMASIN® benefitted all subgroups of patients including those who had disease recurrence during or after neoadjuvant/adjuvant non steroidal AI therapy, those with visceral and bone metastases, as well as those who had prior chemotherapy. The most common adverse events in all age groups were rash, stomatitis, fatigue, diarrhea and nausea and these toxicities were manageable. The authors concluded that AFINITOR® given along with AROMASIN® in the study population can decrease the risk of disease progression by 60% and can double the Clinical Benefit Rate, compared to AROMASIN® alone, even in patients 65 years of age or older, thereby delaying the need for chemotherapy. Beck JT, Hortobagyi GN, Campone M, et al. Breast Cancer Res Treat. 2014; 143: 459–467

