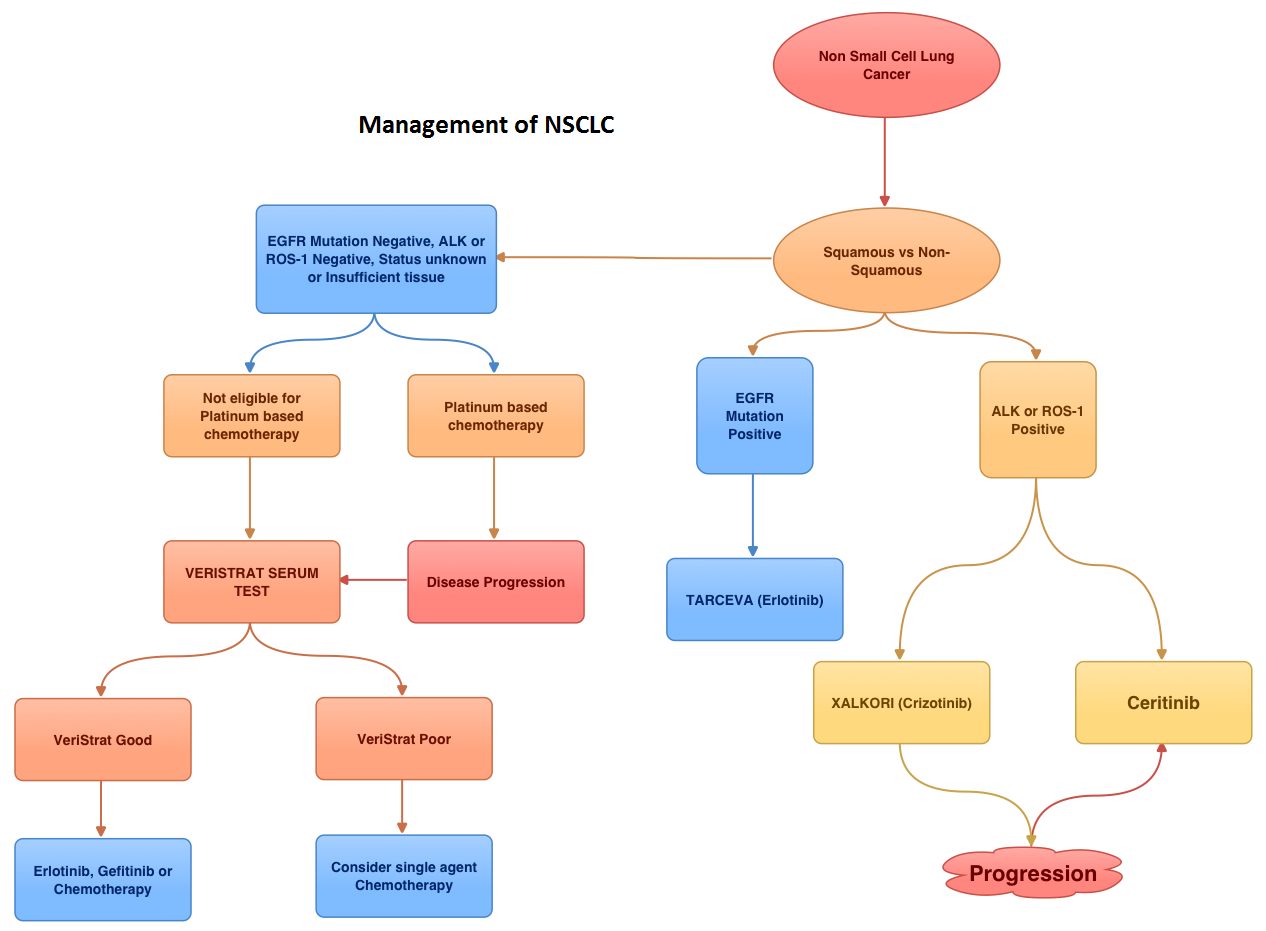
SUMMARY: EML4-ALK (Echinoderm Microtubule associated protein Like 4) – (Anaplastic Lymphoma Kinase) is an aberrant fusion-type oncoprotein and is a tyrosine kinase. This oncoprotein/tyrosine kinase is found in 2-7% of all Non Small Cell Lung Cancers (NSCLC) and is generated due to an inversion in the short arm of chromosome 2. This oncoprotein is more prevalent in patients with adenocarcinoma, who have little or no exposure to tobacco. Tyrosine kinases normally play an important role in cellular proliferation and differentiation. However with point mutations, translocation/rearrangement and amplification of the respective genes, the associated tyrosine kinases can potentially cause malignancy. Such is the case with mutations or translocations of the Anaplastic Lymphoma Kinase gene (ALK). XALKORI® (Crizotinib) is a small molecule Tyrosine Kinase Inhibitor that targets ALK, MET and ROS1 tyrosine kinases. In an open label phase III trial involving 347 patients with locally advanced or metastatic ALK-positive lung cancer who had received one prior platinum based regimen, treatment with XALKORI® significantly improved Progression Free Survival (PFS) and Response Rates (RR). In spite of this initial benefit, patients will however relapse within 12 months, with the average response duration of about 8 months. This has been attributed to acquired mutation within the ALK tyrosine kinase domain, amplification of the ALK fusion gene, subtherapeutic inhibition of ALK tyrosine kinase or activation of other pathways that can cause abnormal cell proliferation. Ceritinib (LDK378) is an oral, small molecule, second generation tyrosine kinase inhibitor of ALK and is 20 times as potent as XALKORI® against ALK. Unlike XALKORI®, Ceritinib does not inhibit MET kinase activity. Based on preclinical data supporting the efficacy of Ceritinib in both XALKORI® sensitive and resistant NSCLC tumors, the authors conducted a study to evaluate the antitumor activity of Ceritinib in patients with advanced NSCLC and other cancers harboring genetic alterations in ALK, in addition to determining the safety, MTD (maximum tolerated dose) and pharmacokinetics of Ceritinib. In this trial, patients who had received prior therapy with one or more ALK inhibitors as well as those with asymptomatic treated or untreated CNS metastases, were eligible to be enrolled. This study had 2 components – a dose escalation phase and an expansion phase. In the dose escalation phase, 59 patients were enrolled and the MTD of Ceritinib was determined to be 750 mg PO daily. In the expansion phase, 71 additional patients were treated for a total of 130 patients (N=59+71). Majority of these patients (94%) had advanced NSCLC. Patients with NSCLC who received at least 400mg of Ceritinib daily (N=114) had an overall response rate (RR) of 58% and median PFS was 7 months. Patients with advanced NSCLC who had received XALKORI® prior to enrollment (N=80) had a RR of 56%. The responses were noted both in patients with tumors harboring resistance mutations in the ALK tyrosine kinase domain as well as those in whom there was no new genetic alterations of ALK. Further, responses were seen in the untreated CNS lesions both in patients who had prior therapy with XALKORI® as well as those who did not. Adverse events were grade 1or 2 and GI related. These included vomiting, diarrhea, elevated aminotransferase levels and hypophosphatemia. The authors concluded that Cerifinib has marked antitumor activity in patients with advanced ALK rearranged NSCLC and in those who had progressed during XALKORI® treatment, regardless of the presence of resistance mutations in the ALK tyrosine kinase domain. Whether Cerifinib should be considered for the first line treatment of advanced ALK rearranged NSCLC, remains to be seen. Shaw AT, Kim D, Mehra R, et al. N Engl J Med 2014; 370:1189-1197