SUMMARY:Prostate cancer is the most common cancer in American men with the exclusion of skin cancer and 1 in 7 men will be diagnosed with prostate cancer during their lifetime. It is estimated that in the United States, about 220,800 new cases of prostate cancer will be diagnosed in 2015 and over 27,000 men will die of the disease. The development and progression of prostate cancer is driven by androgens. Androgen Deprivation Therapy (ADT) has therefore been the cornerstone of treatment of advanced prostate cancer and is the first treatment intervention for hormone sensitive prostate cancer. 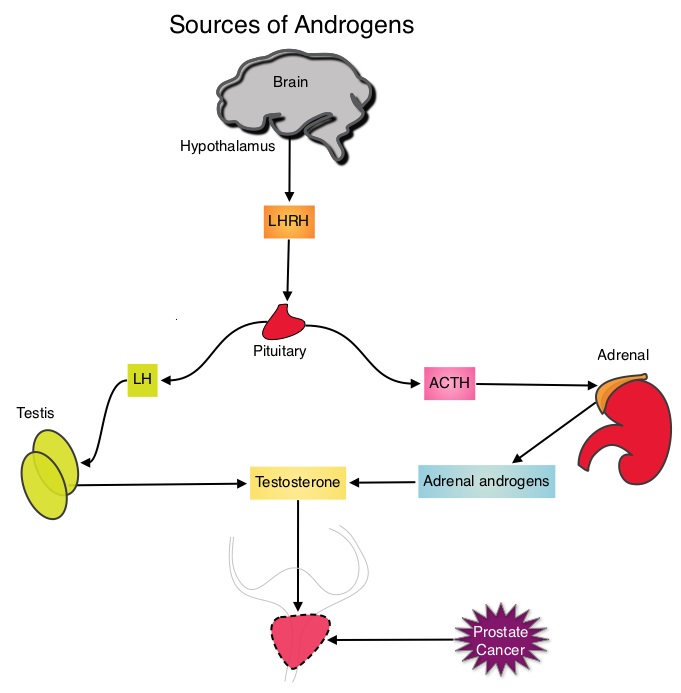 This is accomplished by either surgical castration (bilateral orchiectomy) or medical castration using LHRH (GnRH- Gonadotropin-Releasing Hormone) agonists given along with 2 weeks of first generation anti-androgen agents such as EULEXIN® (Flutamide), CASODEX® (Bicalutamide) or NILANDRON® (Nilutamide), with the anti-androgen agents given to prevent testosterone flare. This large intergroup trial which was developed by the NCIC Clinical Trials Group in collaboration with the Medical Research Council and the National Cancer Institute US Cancer Therapy Evaluation Program, evaluated the benefits of adding Radiation Therapy (RT) to ADT, when compared to ADT alone, in patients with locally advanced prostate cancer. In this study, 1205 patients were randomly assigned to receive either ADT alone (N=602) or ADT plus RT (N=603). Eligible patients included those with T1-2 disease with either Prostate Specific Antigen (PSA) of more than 40 μg/L or PSA of 20-40 μg/L plus Gleason score of 8-10 or patients with T3-4, N0/NX, M0 prostate cancer. ADT consisted of either bilateral orchiectomy or LHRH agonists (plus 2 weeks of oral anti-androgen therapy to prevent testosterone flare), based on patient and physician preference, and ADT was continued for life. RT consisted of a dose of 64-69 Gy given in 35-39 fractions to the prostate gland and pelvis or prostate gland alone. The median age was 70 years and the median follow up was 8 years. Eighty seven percent of patients had T3-4 disease, 63% of patients had a PSA more than 20 μg/L and 18% had a Gleason score of more than 8. The Primary Endpoint was Overall Survival (OS), defined as the time from randomization to death from any cause. Secondary Endpoints included Time To Progression (TTP), improvement in Disease Specific Survival, quality of life and toxicity. The authors had previously reported the interim analysis findings of this intergroup trial and they noted that the addition of RT to ADT significantly improved overall survival, at a median follow up of 6 years (HR= 0.77; P=0.033). In this final analysis, at a median follow up of 8 years, the interim analysis findings were confirmed and the patients assigned to ADT plus RT had significantly improved Overall Survival compared to those who received ADT alone (HR=0.70; P<0.001), with a 30% reduction in the risk of death. Disease Specific Survival was also superior with ADT plus RT compared to ADT alone, with a 54% reduction in deaths from prostate cancer (HR=0.46; P <0 .001). There was a higher incidence of grade 1 and 2 bowel toxicities in patients who received ADT plus RT, but grade 3 bowel toxicities were rare and short term. The authors concluded that this long term follow up data suggests that the addition of Radiation Therapy to Androgen Deprivation Therapy significantly prolongs Overall and Disease Specific Survival, in patients with locally advanced prostate cancer. Final Report of the Intergroup Randomized Study of Combined Androgen-Deprivation Therapy Plus Radiotherapy Versus Androgen-Deprivation Therapy Alone in Locally Advanced Prostate Cancer. Mason MD, Parulekar WR, Sydes MR, et al. J Clin Oncol 2015; 33:2143-2150
This is accomplished by either surgical castration (bilateral orchiectomy) or medical castration using LHRH (GnRH- Gonadotropin-Releasing Hormone) agonists given along with 2 weeks of first generation anti-androgen agents such as EULEXIN® (Flutamide), CASODEX® (Bicalutamide) or NILANDRON® (Nilutamide), with the anti-androgen agents given to prevent testosterone flare. This large intergroup trial which was developed by the NCIC Clinical Trials Group in collaboration with the Medical Research Council and the National Cancer Institute US Cancer Therapy Evaluation Program, evaluated the benefits of adding Radiation Therapy (RT) to ADT, when compared to ADT alone, in patients with locally advanced prostate cancer. In this study, 1205 patients were randomly assigned to receive either ADT alone (N=602) or ADT plus RT (N=603). Eligible patients included those with T1-2 disease with either Prostate Specific Antigen (PSA) of more than 40 μg/L or PSA of 20-40 μg/L plus Gleason score of 8-10 or patients with T3-4, N0/NX, M0 prostate cancer. ADT consisted of either bilateral orchiectomy or LHRH agonists (plus 2 weeks of oral anti-androgen therapy to prevent testosterone flare), based on patient and physician preference, and ADT was continued for life. RT consisted of a dose of 64-69 Gy given in 35-39 fractions to the prostate gland and pelvis or prostate gland alone. The median age was 70 years and the median follow up was 8 years. Eighty seven percent of patients had T3-4 disease, 63% of patients had a PSA more than 20 μg/L and 18% had a Gleason score of more than 8. The Primary Endpoint was Overall Survival (OS), defined as the time from randomization to death from any cause. Secondary Endpoints included Time To Progression (TTP), improvement in Disease Specific Survival, quality of life and toxicity. The authors had previously reported the interim analysis findings of this intergroup trial and they noted that the addition of RT to ADT significantly improved overall survival, at a median follow up of 6 years (HR= 0.77; P=0.033). In this final analysis, at a median follow up of 8 years, the interim analysis findings were confirmed and the patients assigned to ADT plus RT had significantly improved Overall Survival compared to those who received ADT alone (HR=0.70; P<0.001), with a 30% reduction in the risk of death. Disease Specific Survival was also superior with ADT plus RT compared to ADT alone, with a 54% reduction in deaths from prostate cancer (HR=0.46; P <0 .001). There was a higher incidence of grade 1 and 2 bowel toxicities in patients who received ADT plus RT, but grade 3 bowel toxicities were rare and short term. The authors concluded that this long term follow up data suggests that the addition of Radiation Therapy to Androgen Deprivation Therapy significantly prolongs Overall and Disease Specific Survival, in patients with locally advanced prostate cancer. Final Report of the Intergroup Randomized Study of Combined Androgen-Deprivation Therapy Plus Radiotherapy Versus Androgen-Deprivation Therapy Alone in Locally Advanced Prostate Cancer. Mason MD, Parulekar WR, Sydes MR, et al. J Clin Oncol 2015; 33:2143-2150

