SUMMARY: Prostate cancer is the most common cancer in American men with the exclusion of skin cancer and 1 in 7 men will be diagnosed with prostate cancer during their lifetime. It is estimated that in the United States, about 180,890 new cases of prostate cancer will be diagnosed in 2016 and over 26,000 men will die of the disease. The major source of PSA (Prostate Specific Antigen) is the prostate gland and the PSA levels are therefore undetectable within 6 weeks after Radical Prostatectomy. Similarly, following Radiation Therapy, there is a gradual decline in PSA before reaching a post treatment nadir. A detectable PSA level after Radical Prostatectomy, or a rising PSA level following Radiation Therapy, is considered PSA failure or biochemical recurrence. The American Urological Association suggested that a PSA of 0.2 ng/mL or higher after Radical Prostatectomy, defines PSA failure or relapse. A PSA rise 2 ng/ml or more above post Radiation Therapy nadir is considered PSA failure or relapse. Approximately 35% of the patients with prostate cancer will experience PSA only relapse within 10 years of their primary treatment and a third of these patients will develop documented metastatic disease within 8 years following PSA only relapse. Rising PSA is therefore a sign of recurrent disease. The development and progression of prostate cancer is driven by androgens. Androgen Deprivation Therapy (ADT) has therefore been the cornerstone of treatment of advanced prostate cancer and is the first treatment intervention for Hormone Sensitive Prostate Cancer. The appropriate time (immediate versus delayed) to start Androgen Deprivation Therapy in patients with prostate cancer with rising PSA, as the only sign of relapse, has remained unclear. This has been partly due to lack of patient accruals and patient reluctance to be randomized, in these clinical trials.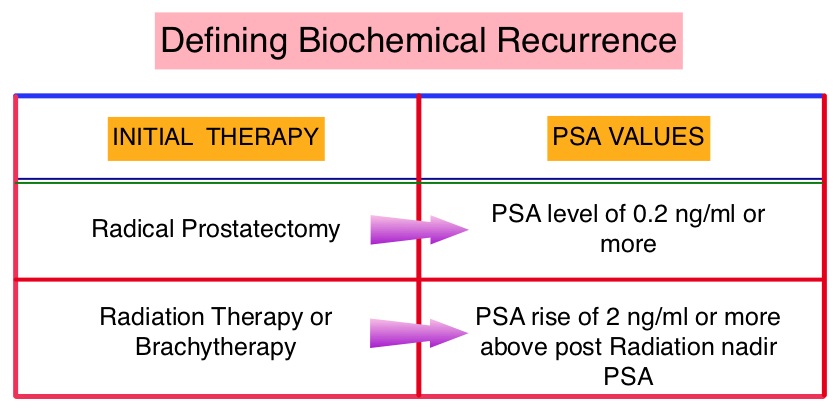
The authors conducted a randomized, prospective, phase III trial, to determine if immediate intervention with Androgen Deprivation Therapy (ADT) would improve Overall Survival (OS), compared with delayed ADT, in prostate cancer patients with PSA relapse, following previous attempted curative therapy (radiotherapy or surgery with or without postoperative radiotherapy) or in those considered not suitable for curative treatment (because of age, comorbidity or locally advanced disease). This analysis combined prostate cancer patients with PSA relapse enrolled in two separate studies. Two hundred and ninety three (N=293) eligible patients were randomly assigned 1:1 to immediate Androgen Deprivation Therapy (N= 142) or delayed ADT (N=151). The primary endpoint was Overall Survival. Secondary endpoints included Cancer-Specific Survival and Time to Clinical Progression. The median follow up was 5 years.
There was a statistically significant improvement in the Overall Survival, with a 45% reduction in the risk for death, for those receiving immediate ADT compared with the delayed treatment group (HR=0.55; P=0.05). Further, with immediate ADT, there was a statistically significant delay in the time to first local progression (HR= 0.51; P=0.001).
The authors concluded that immediate Androgen Deprivation Therapy significantly improved Overall Survival and Time to Clinical Progression for prostate cancer patients with PSA relapse, following immediate intervention with Androgen Deprivation Therapy. This benefit however must be weighed against the risks associated with long term Androgen Deprivation Therapy. Immediate ADT may be appropriate for patients with high risk features at the time of initial diagnosis, who present with early biochemical relapse after initial treatment and have a rapid PSA doubling time (less than 6 months). Timing of androgen-deprivation therapy in patients with prostate cancer with a rising PSA (TROG 03.06 and VCOG PR 01-03 [TOAD]): a randomised, multicentre, non-blinded, phase 3 trial. Duchesne GM, Woo HH, Bassett JK, et al. Lancet Oncol 2016;17:727-737

