SUMMARY: Cancer of the endometrium is the most common cancer of the female reproductive organs in the United States. The American Cancer Society estimates that for 2015, about 54,870 new cases of cancer of the body of the uterus will be diagnosed and 10,170 women will die of the disease. Risk factors include age, factors that influence hormone levels such as obesity and estrogen replacement therapy, family history, diet and exercise, drugs such as Tamoxifen, etc.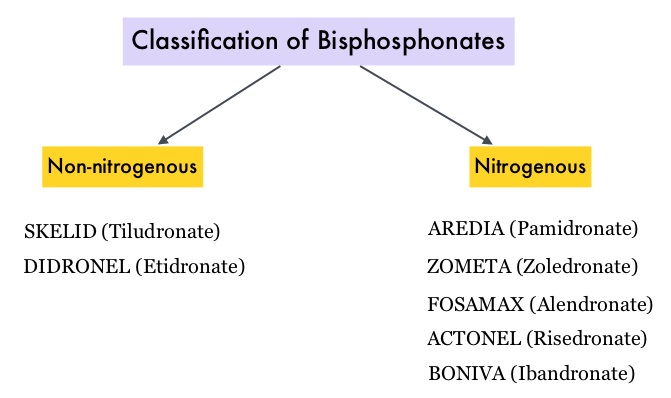 Bisphosphonates such as DIDRONEL® (Etidronate) have been available in the US since the late 1970’s. Bisphosphonates inhibit bone resorption (loss of bone mass) and are indicated for the treatment of osteoporosis and related diseases. The risk of fractures in postmenopausal women with osteoporosis is significantly reduced with the use of bisphosphonates. Several bisphosphonate derivatives have been developed for the treatment of osteoporosis. Aminobisphosphonates such as AREDIA® (Pamidronate) and ZOMETA® (Zoledronic acid) are an integral part of patient management in oncology practice, when bone metastases are initially diagnosed and may have cytostatic, proapoptotic, and antimetastatic properties. An estimated 14.7 million prescriptions for oral bisphosphonates were dispensed in U.S. retail pharmacies in 2012. Previously published studies have shown an inverse relationship between bisphosphonate use and breast cancer risk. Endometrial cancers which are hormone mediated, share risk factors with breast cancer and women with a history of fractures have a lower risk of endometrial cancers.
Bisphosphonates such as DIDRONEL® (Etidronate) have been available in the US since the late 1970’s. Bisphosphonates inhibit bone resorption (loss of bone mass) and are indicated for the treatment of osteoporosis and related diseases. The risk of fractures in postmenopausal women with osteoporosis is significantly reduced with the use of bisphosphonates. Several bisphosphonate derivatives have been developed for the treatment of osteoporosis. Aminobisphosphonates such as AREDIA® (Pamidronate) and ZOMETA® (Zoledronic acid) are an integral part of patient management in oncology practice, when bone metastases are initially diagnosed and may have cytostatic, proapoptotic, and antimetastatic properties. An estimated 14.7 million prescriptions for oral bisphosphonates were dispensed in U.S. retail pharmacies in 2012. Previously published studies have shown an inverse relationship between bisphosphonate use and breast cancer risk. Endometrial cancers which are hormone mediated, share risk factors with breast cancer and women with a history of fractures have a lower risk of endometrial cancers.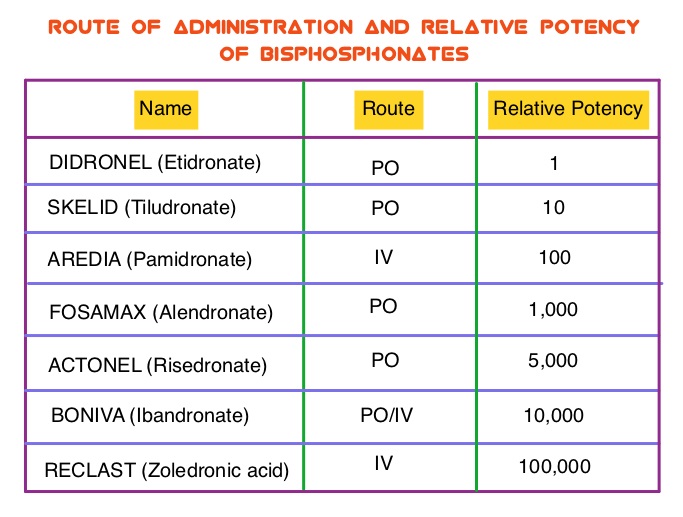 The association between bisphosphonate use and endometrial cancer has remained unclear except for a few, small retrospective studies, which have shown an inverse relationship. To further explore this observation, the authors conducted a large prospective study which included 89,918 postmenopausal women participating in the Women’s Health Initiative (WHI). Following a health information interview conducted at baseline, the use of bisphosphonate was ascertained at baseline and over the follow up period. All women had an intact uterus at the time of enrollment and the most common type of bisphosphonate used was FOSAMAX® (Alendronate). During the study median follow up of 12.5 years, 1,123 women were diagnosed with incident invasive endometrial cancer. Ever use of bisphosphonates was inversely associated with age-adjusted endometrial cancer risk (HR=0.76; P=0.01). There was no evidence of statistically significant interactions with age at baseline, BMI (Body Mass Index), or hip fracture probability score. In this large prospective cohort of postmenopausal women, the authors concluded that bisphosphonate use was associated with a statistically significant reduction in endometrial cancer risk, in postmenopausal women. Oral Bisphosphonate Use and Risk of Postmenopausal Endometrial Cancer. Newcomb PA, Passarelli MN, Phipps AI, et al. J Clin Oncol 2015; 33: 1186-1190
The association between bisphosphonate use and endometrial cancer has remained unclear except for a few, small retrospective studies, which have shown an inverse relationship. To further explore this observation, the authors conducted a large prospective study which included 89,918 postmenopausal women participating in the Women’s Health Initiative (WHI). Following a health information interview conducted at baseline, the use of bisphosphonate was ascertained at baseline and over the follow up period. All women had an intact uterus at the time of enrollment and the most common type of bisphosphonate used was FOSAMAX® (Alendronate). During the study median follow up of 12.5 years, 1,123 women were diagnosed with incident invasive endometrial cancer. Ever use of bisphosphonates was inversely associated with age-adjusted endometrial cancer risk (HR=0.76; P=0.01). There was no evidence of statistically significant interactions with age at baseline, BMI (Body Mass Index), or hip fracture probability score. In this large prospective cohort of postmenopausal women, the authors concluded that bisphosphonate use was associated with a statistically significant reduction in endometrial cancer risk, in postmenopausal women. Oral Bisphosphonate Use and Risk of Postmenopausal Endometrial Cancer. Newcomb PA, Passarelli MN, Phipps AI, et al. J Clin Oncol 2015; 33: 1186-1190

