The FDA on July 31, 2023, approved JEMPERLI® with Carboplatin and Paclitaxel, followed by single-agent JEMPERLI®, for primary advanced or recurrent Endometrial Cancer (EC) that is MisMatch Repair deficient (dMMR), as determined by an FDA-approved test, or MicroSatellite Instability-High (MSI-H). JEMPERLI® is a product of GlaxoSmithKline.
Tag: Endometrial Cancer
Hair Relaxer Use and Risk of Uterine Cancer
SUMMARY: The American Cancer Society estimates that approximately 66,200 new cases of uterine cancer will be diagnosed in 2023 and about 13,030 will die of the disease. Uterine cancer is the second most prevalent gynecologic cancer in women worldwide, and its incidence has been increasing. Risk factors include age, factors that influence hormone levels such as obesity and estrogen replacement therapy, Type 2 diabetes, family history, diet and exercise, drugs such as Tamoxifen, and delayed menopause. Exposure to excess estrogen and a hormonal imbalance of estrogen and progesterone have been identified as key risk factors for uterine cancer. Further, synthetic estrogenic compounds such as endocrine-disrupting chemicals have been implicated, because of their ability to alter hormonal actions.
It is estimated that up to 95% of self-identified adult African American women in the U.S. report ever use of hair relaxers. Chemical straighteners or relaxers are commonly used by Black women to straighten curly or tightly coiled hair mostly due to societal pressure to straighten hair. As a result, chemical hair relaxers are heavily marketed to Black women. These straighteners have been found to have potentially harmful toxicants such as phthalates, parabens, cyclosiloxanes and metals that may act as carcinogens or endocrine disruptors, and may release formaldehyde when heated. These products when left on the hair longer than advised can injure the scalp, making it easier to absorb the chemicals. High concentrations of metabolites of phthalates and parabens have been detected in urine samples from women who use chemical hair relaxers.
Endocrine disrupting chemicals may cause hormonal imbalance through either estrogenic or anti-estrogenic activity and has been associated with earlier puberty, infertility, and uterine fibroids. A study published in the International Journal of Cancer in 2020 found that, women who used chemical hair straighteners more than 6 times a year, had about a 30% higher risk of breast cancer. In a study published in the journal Carcinogenesis in 2021 and in the Journal of the National Cancer Institute in 2022 (The Sister Study), women who used chemical hair straighteners more than 4 times a year, were twice as likely to develop ovarian cancer and more than twice as likely to develop uterine cancer as compared to women who did not use chemical hair straighteners. However in the Sister Study there were only 17 exposed cases among Black women. The researchers in this study sought to evaluate the possible association of hair relaxer use with uterine cancer risk in a large prospective cohort of Black women.
The US-based Black Women’s Health Study (BWHS) included 44,798 Black women with an intact uterus and no prior history of cancer, between the ages of 21 and 69, who were followed from 1997 until 2019. All participants completed a self-administered questionnaire about their personal and family medical history as well as behavioral and other factors such as medication use, cigarette smoking, and diet. The researchers used adjusted multivariable Cox proportional hazards regression models, to estimate Hazard Ratios (HRs) and 95% Confidence Intervals (CIs) for associations of hair relaxer use with risk of uterine cancer. The participants were followed for up to 22 years, and the rates of uterine cancer among women who reported frequent or long-term use of hair relaxers were compared to rates among women who never or rarely used them.
It was noted that compared to women who never used hair relaxers or used them infrequently (less than 4 years and 1-2 times or less/year), the Hazard Ratio for uterine cancer associated with heavy use (15 years or more and at least 5 times/year) was 1.18. However, among postmenopausal women, the rates of uterine cancer were statistically significantly higher for those who commonly used hair relaxers even after adjustment for other potential risk factors. In postmenopausal women compared to never/light use, the Hazard Ratio for moderate use was 1.60, the Hazard Ratio for heavy use was 1.64, and the Hazard Ratio for 20 or more years of use regardless of frequency was 1.71. Postmenopausal women who reported using hair relaxers more than twice a year, or for more than five years had a greater than 50% increased risk of uterine cancer, compared to women who never or rarely used hair relaxers.
It was concluded from this large study that long-term use of chemical hair relaxers was associated with increased risk of uterine cancer among postmenopausal women, even after adjustment for other potential risk factors, but not among premenopausal women. These findings suggest that hair relaxer use may be a potentially modifiable risk factor for uterine cancer and the researchers hope these results will raise awareness of the potential toxic effects of these products and promote efforts to reduce exposure.
Hair relaxer use and risk of uterine cancer in the Black Women’s Health Study. Bertrand KA, Delp L, Coogan PF, et al. Environmental Research 2023. Volume 239, Part 1, 15 December 2023, 117228. https://doi.org/10.1016/j.envres.2023.117228
JEMPERLI® (Dostarlimab-gxly)
The FDA on February 9, 2023, approved JEMPERLI® for adult patients with MisMatch Repair deficient (dMMR) recurrent or advanced Endometrial cancer, as determined by an FDA-approved test, that has progressed on or following a prior platinum-containing regimen in any setting and are not candidates for curative surgery or radiation. JEMPERLI® is a product of GlaxoSmithKline LLC.
Selinexor Maintenance in Advanced Endometrial Cancer
SUMMARY: The American Cancer Society estimates that approximately 66,200 new cases of uterine cancer will be diagnosed in 2023 and about 13,030 individuals will die of the disease. Endometrial carcinoma is the second most prevalent gynecologic cancer in women worldwide, and its incidence has been increasing. Risk factors include age, factors that influence hormone levels such as obesity and estrogen replacement therapy, Type 2 diabetes, family history, diet and exercise, drugs such as Tamoxifen, and delayed menopause. Patients with advanced or recurrent endometrial cancer are often treated with a combination of Carboplatin and Paclitaxel. Treatment options following failure of first-line therapy for this patient group however are limited, with single agent response rates of 10-15% and 5-year survival rates of approximately 17%. There are no approved therapies in the maintenance setting for patients with advanced or recurrent endometrial cancer.
It is estimated that of the endometrial carcinoma molecular subtypes, TP53 wild-type tumors represent 75% of the newly diagnosed endometrial carcinoma and 50% of advanced/recurrent tumors. There are no specific targeted therapies available for patients with TP53 wild-type endometrial carcinoma.
Exportin 1 (XPO1) is an important nuclear export protein overexpressed in endometrial cancers. High XPO1 levels facilitate increased nuclear export of tumor suppressor proteins such as P53, P73, IkB and FOXO3a, pRb, BRCA1, as well as growth regulators such as Glucocorticoid Receptor and oncoprotein mRNA. This enables cancer cells to escape tumor suppressor protein mediated cell cycle arrest and apoptosis.
Selinexor (XPOVIO®) is first-in-class, oral selective XPO1 inhibitor that reactivates the tumor suppressor proteins by preventing nuclear transport and inhibits oncoprotein translation. Selinexor is approved in the US for the treatment of patients with Multiple Myeloma and Diffuse Large B-Cell Lymphoma. Selinexor demonstrated antitumor activity among patients with endometrial carcinoma in Phase I and II trials.
SIENDO/EC-042 is a Phase III randomized, double-blind, placebo-controlled study designed to evaluate the efficacy and safety of Selinexor as maintenance therapy in patients with TP53 wild-type advanced or recurrent endometrial carcinoma who have achieved a Partial or Complete Response after completing at least 12 weeks of platinum combination chemotherapy with or without immunotherapy for primary Stage IV or recurrent disease. Comprehensive tissue-based genomic profiling testing was performed, to identify and enroll patients whose tumors were TP53 wild-type. In this study, 263 eligible patients were randomly assigned in a 2:1 ratio to receive Selinexor 60 mg orally once weekly (N=174) or placebo weekly (N=89). The prespecified TP53 wild-type subgroup included 113 women assigned to Selinexor (N=77) or Placebo (N=36). The median age in the TP53 wild-type subgroup was 63 years and most patients had endometrioid histology and MSS/MMR proficient tumors. Patients were stratified based on whether they had primary Stage IV or recurrent disease, as well as Partial or Complete response to platinum combination chemotherapy before they were started on maintenance therapy with Selinexor. The Primary endpoint was investigator-assessed Progression Free Survival (PFS), with exploratory endpoints including histologic subtype and molecular subclassifications. Secondary end points included PFS by Blinded Independent Central Review, Overall Survival (OS), time to first subsequent therapy, and Health-Related Quality of Life. The primary analysis of Selinexor maintenance therapy showed improvements in median PFS for the intent-to-treat (ITT) population but were not clinically meaningful.
However, an exploratory analysis of a pre-specified subgroup of patients with TP53 wild-type endometrial cancer showed significant findings. At a median follow up of 25.3 months, it was noted that patients with TP53 wild-type tumors receiving Selinexor maintenance therapy had a median PFS of 27.4 months compared with 5.2 months in the placebo group, representing a 58% decrease in the risk of disease progression (HR=0.42; P=0.0003). This efficacy was observed regardless of MSS/MSI status, but women in the TP53 wild-type subgroup who had MSS/MMR proficient tumors demonstrated the greatest PFS benefit with Selinexor. In the subgroup with TP53 mutations/aberrations, the median PFS was shorter with Selinexor (4.2 months versus 5.4 months with placebo; HR=1.34: P=0.92). The most common adverse events with Selinexor in the TP53 wild-type subgroup included nausea (90.8%), vomiting (60.5%) and diarrhea (39.5%). Grade 3-4 events included neutropenia (18.4%) and thrombocytopenia (9.2%).
The authors concluded that TP53 status is a robust prognostic biomarker for endometrial carcinoma and Selinexor maintenance in TP53 wild-type endometrial carcinoma demonstrated durable Progression Free Survival benefit in a pre-specified subgroup analysis, and offers the potential to prolong response to prior systemic therapy.
Long-term follow up of selinexor maintenance in patients with TP53wt advanced or recurrent endometrial cancer: A pre-specified subgroup analysis from the phase 3 ENGOT-EN5/GOG-3055/SIENDO study. Slomovitz B. American Society of Clinical Oncology Plenary Series. July 25, 2023; virtual; abstract 427956.
KEYTRUDA® (Pembrolizumab)
The FDA on March 21, 2022, approved KEYTRUDA® (Pembrolizumab) as a single agent, for patients with advanced endometrial carcinoma that is MicroSatellite Instability-High (MSI-H) or MisMatch Repair deficient (dMMR), as determined by an FDA-approved test, who have disease progression following prior systemic therapy in any setting, and who are not candidates for curative surgery or radiation. KEYTRUDA® is a product of Merck & Co., Inc.
FDA Approves Single Agent KEYTRUDA® for Advanced Endometrial Carcinoma
SUMMARY: The FDA on March 21, 2022 approved KEYTRUDA® (Pembrolizumab) as a single agent, for patients with advanced endometrial carcinoma that is MicroSatellite Instability-High (MSI-H) or MisMatch Repair deficient (dMMR), as determined by an FDA-approved test, who have disease progression following prior systemic therapy in any setting, and who are not candidates for curative surgery or radiation. The FDA also approved VENTANA MMR RxDx Panel (Ventana Medical Systems/Roche Tissue Diagnostics) as a companion diagnostic device to select patients with dMMR in solid tumors that are eligible for treatment with KEYTRUDA® The FDA previously approved the FoundationOne CDx (F1CDx, Foundation Medicine, Inc.) as a companion diagnostic device to select patients with MSI-H in solid tumors that are eligible for treatment with KEYTRUDA®.
The American Cancer Society estimates that approximately 65,950 new cases of uterine cancer will be diagnosed in 2022 and about 12,550 individuals will die of the disease. Endometrial carcinoma is the second most prevalent gynecologic cancer in women worldwide, and its incidence has been increasing. Risk factors include age, factors that influence hormone levels such as obesity and estrogen replacement therapy, family history, diet and exercise, drugs such as Tamoxifen, etc. Patients with advanced or recurrent endometrial cancer are often treated with a combination of Carboplatin and Paclitaxel. Treatment options following failure of first-line therapy for this patient group however are limited, with single agent response rates of 10-15% and 5-year survival rates of approximately 17%.
The DNA MisMatchRepair (MMR) system is responsible for molecular surveillance and works as an editing tool that identifies errors within the microsatellite regions of DNA and removes them. Defective MMR system leads to MSI (Micro Satellite Instability) and hypermutation, with the expression of tumor-specific neoantigens at the surface of cancer cells, triggering an increase in CD3-positive, CD8-positive, and Programmed Death-1 (PD-1) expressing Tumor Infiltrating Lymphocytes and Programmed Death Ligand-1 (PD-L1) expressing intraepithelial and peritumoral immune cells, compared with MicroSatellite Stable cancers. This results in an enhanced antitumor immune response.
MSI is therefore a hallmark of defective/deficient DNA MisMatchRepair (dMMR) system. Defective MMR can be a sporadic or heritable event and can manifest as a germline mutation occurring in MMR genes including MLH1, MSH2, MSH6 and PMS2. This produces Lynch Syndrome often called Hereditary Nonpolyposis Colorectal Carcinoma-HNPCC, an Autosomal Dominant disorder that is often associated with a high risk for Colorectal and Endometrial carcinoma, as well as several other malignancies including Ovary, Stomach, Small bowel, Hepatobiliary tract, Brain and Skin. MSI is a hallmark of Lynch Syndrome-associated cancers. MSI high tumors tend to have better outcomes and this has been attributed to the abundance of Tumor Infiltrating Lymphocytes in these tumors from increase immunogenicity. These tumors therefore are susceptible to blockade with Immune Checkpoint Inhibitors.
MSI testing is performed using a PCR or NGS based assay and MSI-High refers to instability at 2 or more of the 5 mononucleotide repeat markers and MSI-Low refers to instability at 1 of the 5 markers. Patients are considered Micro Satellite Stable (MSS) if no instability occurs. MSI-L and MSS are grouped together because MSI-L tumors are uncommon and behave similar to MSS tumors. Tumors considered MSI-H have deficiency of one or more of the DNA MMR genes. MMR gene deficiency can be detected by ImmunoHistoChemistry (IHC). NCCN Guidelines recommend MMR or MSI testing for all patients with a history of Colon or Rectal cancer. Unlike Colorectal and Endometrial cancer, where MSI-H/dMMR testing is routinely undertaken, the characterization of Lynch Syndrome across heterogeneous MSI-H/dMMR tumors is unknown.
KEYTRUDA® (Pembrolizumab) is a fully humanized, Immunoglobulin G4, anti-PD-1, monoclonal antibody, that binds to the PD-1 receptor and blocks its interaction with ligands PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the tumor-specific effector T cells. The FDA in 2017 granted accelerated approval to KEYTRUDA® for patients with advanced MSI-High or dMMR solid tumors, that have progressed following prior treatment, and who have no satisfactory alternative treatment options. This has led to routine MSI-H/dMMR testing in advanced solid tumors. The FDA in 2021 also approved KEYTRUDA® in combination with the multireceptor Tyrosine Kinase Inhibitor LENVIMA® (Lenvatinib) for patients with advanced endometrial carcinoma, irrespective of tumor MSI status based on the KEYNOTE-146 study.
KEYNOTE-158 is a multicenter, nonrandomized, open-label, multicohort, Phase II trial of KEYTRUDA® evaluating predictive biomarkers, in patients with advanced unresectable and/or metastatic solid tumors, who had progressed on standard of care therapy. The present FDA approval was based on the results from a total of 90 patients with MSI-H/dMMR endometrial cancer, who were enrolled in cohort D (11 patients) and cohort K (79 patients) of KEYNOTE-158 trial. This group of previously treated patients received KEYTRUDA® 200 mg IV once every 3 weeks for 35 cycles. The median patient age was 64 years, 48% had received 2 or more lines of prior therapy, and the majority of patients (68%) had received prior radiation therapy. The median duration of treatment was 8.3 months. The Primary end point was Objective Response Rate (ORR) by independent central radiologic review. Secondary end points included Duration of Response, Progression Free Survival (PFS), Overall Survival (OS), and Safety.
The Objective Response Rate was 48%, and median Duration of Response was not reached after a median follow up of 42.6 months. The median PFS was 13.1 months, and median Overall Survival was Not Reached. No new safety signals were identified and the immune-mediated adverse events or infusion reactions occurred in 28% of patients and 7% were Grades 3-4, with no fatal events.
It was concluded that KEYTRUDA® demonstrated robust and durable antitumor activity with manageable toxicity in patients with advanced MSI-H/dMMR endometrial cancer, and should be considered as a treatment option for patients with advanced MSI-H/dMMR endometrial cancer, following failure on prior therapy.
Pembrolizumab in Patients With Microsatellite Instability–High Advanced Endometrial Cancer: Results From the KEYNOTE-158 Study. O’Malley DM, Bariani GM, Cassier PA, et al. DOI: 10.1200/JCO.21.01874 Journal of Clinical Oncology 40, no. 7 (March 01, 2022) 752-761. Published online January 06, 2022.
KEYTRUDA® (Pembrolizumab) in combination with LENVIMA® (Lenvatinib)
The FDA on July 21, 2021 approved KEYTRUDA® in combination with LENVIMA® for patients with advanced Endometrial carcinoma that is not MicroSatellite Instability-High (MSI-H) or MisMatch Repair deficient (dMMR), who have disease progression following prior systemic therapy in any setting, and are not candidates for curative surgery or radiation. KEYTRUDA® is a product of Merck & Co. and LENVIMA® is a product of Eisai Co., Ltd.
JEMPERLI® (Dostarlimab-gxly)
The FDA on April 22, 2021 granted accelerated approval to JEMPERLI® for adult patients with MisMatch Repair deficient (dMMR) recurrent or advanced Endometrial cancer, as determined by an FDA-approved test, that has progressed on or following a prior Platinum-containing regimen. JEMPERLI® is a product of GlaxoSmithKline LLC.
KEYTRUDA® (Pembrolizumab) and LENVIMA® (Lenvatinib)
The FDA on September 17, 2019 granted accelerated approval to the combination of KEYTRUDA® and LENVIMA® for the treatment of patients with advanced Endometrial carcinoma that is not MicroSatellite Instability High (MSI-H) or MisMatch Repair deficient (dMMR), and who have disease progression following prior systemic therapy but are not candidates for curative surgery or radiation. KEYTRUDA® is a product of Merck & Co and LENVIMA® is a product of Eisai Inc.
Oral Bisphosphonates May Decrease the Risk of Postmenopausal Endometrial Cancer
SUMMARY: Cancer of the endometrium is the most common cancer of the female reproductive organs in the United States. The American Cancer Society estimates that for 2015, about 54,870 new cases of cancer of the body of the uterus will be diagnosed and 10,170 women will die of the disease. Risk factors include age, factors that influence hormone levels such as obesity and estrogen replacement therapy, family history, diet and exercise, drugs such as Tamoxifen, etc.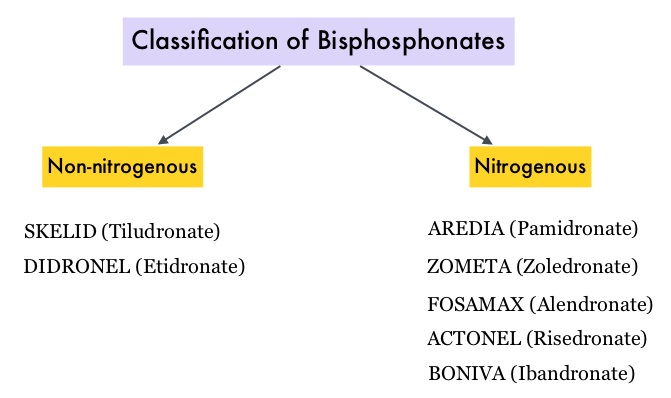 Bisphosphonates such as DIDRONEL® (Etidronate) have been available in the US since the late 1970’s. Bisphosphonates inhibit bone resorption (loss of bone mass) and are indicated for the treatment of osteoporosis and related diseases. The risk of fractures in postmenopausal women with osteoporosis is significantly reduced with the use of bisphosphonates. Several bisphosphonate derivatives have been developed for the treatment of osteoporosis. Aminobisphosphonates such as AREDIA® (Pamidronate) and ZOMETA® (Zoledronic acid) are an integral part of patient management in oncology practice, when bone metastases are initially diagnosed and may have cytostatic, proapoptotic, and antimetastatic properties. An estimated 14.7 million prescriptions for oral bisphosphonates were dispensed in U.S. retail pharmacies in 2012. Previously published studies have shown an inverse relationship between bisphosphonate use and breast cancer risk. Endometrial cancers which are hormone mediated, share risk factors with breast cancer and women with a history of fractures have a lower risk of endometrial cancers.
Bisphosphonates such as DIDRONEL® (Etidronate) have been available in the US since the late 1970’s. Bisphosphonates inhibit bone resorption (loss of bone mass) and are indicated for the treatment of osteoporosis and related diseases. The risk of fractures in postmenopausal women with osteoporosis is significantly reduced with the use of bisphosphonates. Several bisphosphonate derivatives have been developed for the treatment of osteoporosis. Aminobisphosphonates such as AREDIA® (Pamidronate) and ZOMETA® (Zoledronic acid) are an integral part of patient management in oncology practice, when bone metastases are initially diagnosed and may have cytostatic, proapoptotic, and antimetastatic properties. An estimated 14.7 million prescriptions for oral bisphosphonates were dispensed in U.S. retail pharmacies in 2012. Previously published studies have shown an inverse relationship between bisphosphonate use and breast cancer risk. Endometrial cancers which are hormone mediated, share risk factors with breast cancer and women with a history of fractures have a lower risk of endometrial cancers.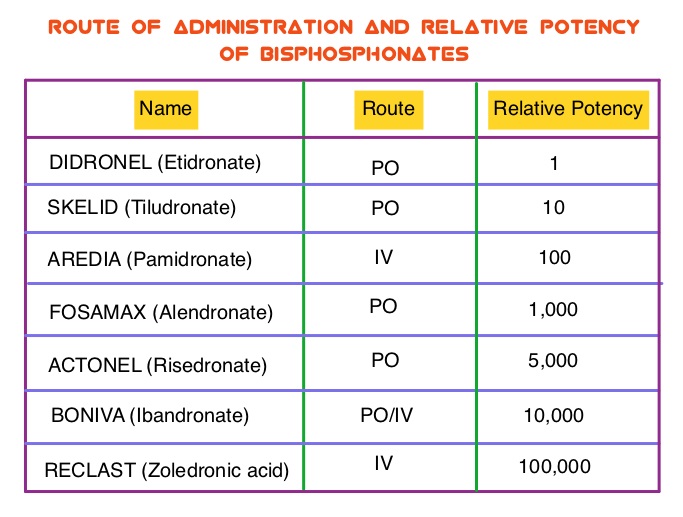 The association between bisphosphonate use and endometrial cancer has remained unclear except for a few, small retrospective studies, which have shown an inverse relationship. To further explore this observation, the authors conducted a large prospective study which included 89,918 postmenopausal women participating in the Women’s Health Initiative (WHI). Following a health information interview conducted at baseline, the use of bisphosphonate was ascertained at baseline and over the follow up period. All women had an intact uterus at the time of enrollment and the most common type of bisphosphonate used was FOSAMAX® (Alendronate). During the study median follow up of 12.5 years, 1,123 women were diagnosed with incident invasive endometrial cancer. Ever use of bisphosphonates was inversely associated with age-adjusted endometrial cancer risk (HR=0.76; P=0.01). There was no evidence of statistically significant interactions with age at baseline, BMI (Body Mass Index), or hip fracture probability score. In this large prospective cohort of postmenopausal women, the authors concluded that bisphosphonate use was associated with a statistically significant reduction in endometrial cancer risk, in postmenopausal women. Oral Bisphosphonate Use and Risk of Postmenopausal Endometrial Cancer. Newcomb PA, Passarelli MN, Phipps AI, et al. J Clin Oncol 2015; 33: 1186-1190
The association between bisphosphonate use and endometrial cancer has remained unclear except for a few, small retrospective studies, which have shown an inverse relationship. To further explore this observation, the authors conducted a large prospective study which included 89,918 postmenopausal women participating in the Women’s Health Initiative (WHI). Following a health information interview conducted at baseline, the use of bisphosphonate was ascertained at baseline and over the follow up period. All women had an intact uterus at the time of enrollment and the most common type of bisphosphonate used was FOSAMAX® (Alendronate). During the study median follow up of 12.5 years, 1,123 women were diagnosed with incident invasive endometrial cancer. Ever use of bisphosphonates was inversely associated with age-adjusted endometrial cancer risk (HR=0.76; P=0.01). There was no evidence of statistically significant interactions with age at baseline, BMI (Body Mass Index), or hip fracture probability score. In this large prospective cohort of postmenopausal women, the authors concluded that bisphosphonate use was associated with a statistically significant reduction in endometrial cancer risk, in postmenopausal women. Oral Bisphosphonate Use and Risk of Postmenopausal Endometrial Cancer. Newcomb PA, Passarelli MN, Phipps AI, et al. J Clin Oncol 2015; 33: 1186-1190
