SUMMARY: Lung cancer is the second most common cancer in both men and women and accounts for about 14% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2018 about 234,030 new cases of lung cancer will be diagnosed and over 154,050 patients will die of the disease. Lung cancer is the leading cause of cancer-related mortality in the United States. Non Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers. Of the three main subtypes of Non Small Cell Lung Cancer (NSCLC), approximately 30% are Squamous Cell Carcinomas (SCC), 40% are Adenocarcinomas, and 10% are Large cell carcinomas. Non Small Cell Lung Cancer patients with Squamous Cell histology have been a traditionally hard- to-treat, patient group, and less than 15% of patients with advanced Squamous NSCLC survive a year after diagnosis and less than 5% of patients survive for five years or longer.
KEYTRUDA® (Pembrolizumab) is a fully humanized, Immunoglobulin G4, anti-PD-1, monoclonal antibody, that binds to the PD-1 receptor and blocks its interaction with ligands PD-L1 and PD-L2. It thereby reverses the PD-1 pathway-mediated inhibition of the immune response and unleashes the tumor-specific effector T cells. The FDA approved KEYTRUDA® for the first-line treatment of advanced NSCLC with high PD-L1 expression (Tumor Proportion Score of 50% or more), as well as in combination with Pemetrexed and Carboplatin, as first-line treatment of patients with metastatic nonsquamous NSCLC and for previously treated advanced NSCLC with a PD-L1 Tumor Proportion Score of 1% or more. Currently, KEYTRUDA® currently is the only FDA approved immunotherapy for initial treatment of NSCLC as monotherapy (KEYNOTE-024) or in combination with chemotherapy (KEYNOTE-189).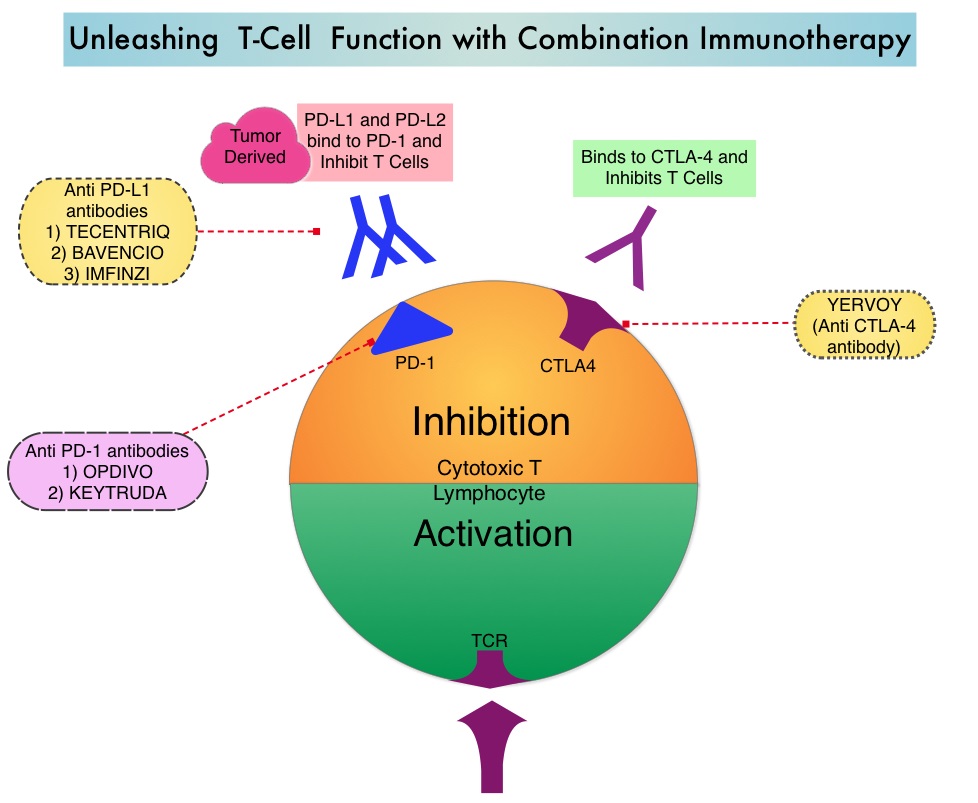
KEYNOTE-407 is a global, double-blind, placebo-controlled, phase 3 trial which compared KEYTRUDA® plus chemotherapy with placebo plus chemotherapy in patients with squamous NSCLC of any level of PD-L1 expression. In this study, 559 patients with untreated metastatic, squamous NSCLC were randomly assigned, in a 1:1 ratio to receive KEYTRUDA® 200 mg IV along with Carboplatin AUC 6 and either TAXOL® (Paclitaxel) 200 mg/m2 IV or ABRAXANE® (nab-paclitaxel) 100 mg/m2 IV days 1, 8 and 15. every 3 weeks for 4 cycles (N=278) or placebo with the same chemotherapy regimen for 4 cycles (N=281). Patients in the experimental arm following the first 4 cycles continued KEYTRUDA® every 3 weeks, for an additional 31 cycles, whereas the control group received placebo. Patients in the placebo-combination group were eligible to cross over to receive KEYTRUDA® monotherapy and 42.8% of patients assigned to the placebo plus chemotherapy group who discontinued chemotherapy went on to receive subsequent anti PD-1 or anti PD-L1 therapy and 75 patients received KEYTRUDA® monotherapy as part of in-study crossover. Patients were stratified according to the PD-L1 Tumor Proportion Score (1% or less versus more than 1%), choice of Taxane (Paclitaxel versus nab-Paclitaxel), and geographic region of enrollment. Tumor Proportion Score is the percentage of tumor cells with membranous PD-L1 staining, with less than 1% indicating PD-L1 negative score. Both treatment groups were well balanced. The co-Primary end points were Overall Survival and Progression Free Survival and the Secondary end points included Response Rate, Duration of Response and Safety. The effects of PD-L1 expression on Overall Survival (OS), Progression Free Survival (PFS), and Objective Response Rate (ORR) were prespecified exploratory end points.
At the second interim analysis, after a median follow-up of 7.8 months, the median Overall Survival was 15.9 months in the KEYTRUDA® combination group and 11.3 months in the placebo combination group (HR=0.64; P<0.001). This meant that there was a 36% reduction in the risk of death with the addition of KEYTRUDA® to chemotherapy. This OS benefit was consistently seen regardless of the level of PD-L1 expression. The median PFS was 6.4 months in the KEYTRUDA® combination group and 4.8 months in the placebo combination group (HR=0.56; P<0.001) and this suggested that the risk of disease progression or death was 44% lower with the addition of KEYTRUDA® to chemotherapy. The PFS benefit with the addition of KEYTRUDA® to chemotherapy was observed in all prespecified subgroups with incremental improvements noted with increasing PD-L1 Tumor Proportion Score. The Objective Response Rate was also significantly higher in the KEYTRUDA® chemotherapy group compared to the placebo chemotherapy group (59.4% versus 38%; P=0.0004), with a median time to response of 1.4 months and median Duration of Response of 7.7 months versus 4.8 months, respectively. Grade 3 or higher adverse events were similar in both treatment groups. Treatment discontinuation due to adverse events was more frequent in the KEYTRUDA® combination group (13.3% versus 6.4%).
It was concluded that inpatients with previously untreated metastatic, squamous NSCLC, the addition of KEYTRUDA® to chemotherapy resulted in significantly longer Overall Survival and Progression Free Survival than chemotherapy alone, and should become a new standard of care for squamous NSCLC. Pembrolizumab plus Chemotherapy for Squamous Non–Small-Cell Lung Cancer. Paz-Ares L, Luft A, Vicente D, et al. for the KEYNOTE-407 Investigators. September 25, 2018. DOI: 10.1056/NEJMoa1810865

