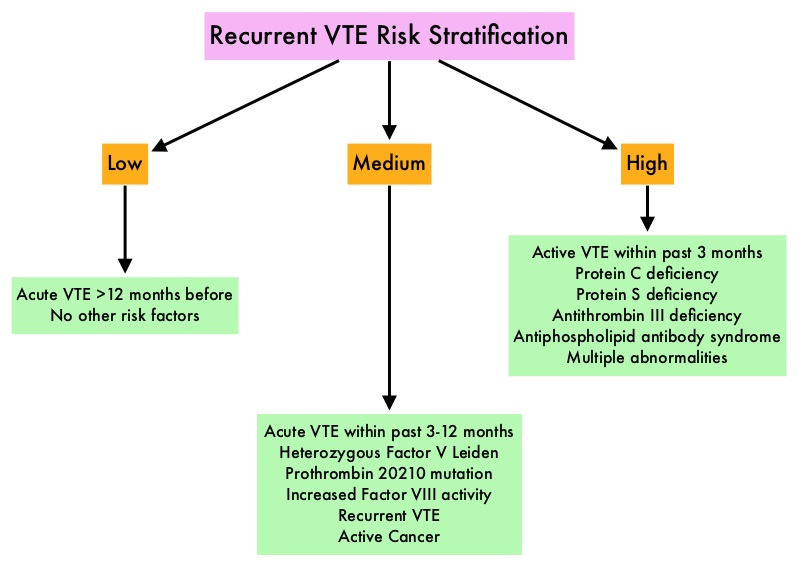
SUMMARY: The FDA on October 16, 2015, granted accelerated approval to Idarucizumab (PRAXBIND®) for the treatment of patients treated with Dabigatran (PRADAXA®), when reversal of the anticoagulant effects of PRADAXA® is needed for emergency surgery/urgent procedures, or in life-threatening or uncontrolled bleeding. There are presently four New Oral Anticoagulants approved in the United States for the treatment of Venous ThromboEmbolism. They include PRADAXA® (Dabigatran), which is a direct thrombin inhibitor and XARELTO® (Rivaroxaban), ELIQUIS® (Apixaban), SAVAYSA® (Endoxaban), which are Factor Xa inhibitors. Compared to COUMADIN® (Warfarin), the New Oral Anticoagulants have a rapid onset of action, wider therapeutic window, shorter half-lives (7-14 hours in healthy individuals), no laboratory monitoring and fixed dosing schedule. The half life of these agents can however be prolonged in those with renal insufficiency. In several clinical studies, these New Oral Anticoagulants have been shown to reduce the rate of major bleeding by 28% and the rates of intracranial and fatal hemorrhage by 50%, when compared to COUMADIN®. Unlike bleeding caused by COUMADIN®, which can be reversed using Vitamin K or Fresh Frozen Plasma, there are no specific agents presently available, for reversing bleeding caused by the New Oral Anticoagulants or for stopping the anticoagulant effects of these drugs, in patients who need urgent surgical intervention.
To address this concern, the RE-VERSE AD study was conducted to evaluate the efficacy and safety of Idarucizumab, to reverse the anticoagulant effects of PRADAXA®, in patients who had serious bleeding (Group A) or required an urgent surgical procedure (Group B). PRAXBIND® (Idarucizumab) is a humanized monoclonal antibody fragment with high affinity for both free and thrombin-bound PRADAXA® and neutralizes the anticoagulant activity of PRADAXA®. In this prospective cohort study, results from the first 90 patients enrolled in the study were presented (Interim analysis). Group A included 51 patients and Group B included 39 patients and PRAXBIND® 5 grams IV was administered, to reverse the anticoagulant effects of PRADAXA®. The median age was 76 years and the median creatinine clearance was 58 ml/minute. The primary end point was the maximum percentage reversal of the anticoagulant effect of PRADAXA® within 4 hours after the administration of PRADAXA®, based on the dilute thrombin time or ecarin clotting time. An important secondary end point was the restoration of hemostasis. Among the patients with an elevated dilute thrombin time and ecarin clotting time at baseline, the median maximum percentage reversal was 100% and this benefit was seen within minutes after the first infusion with PRAXBIND®. The median time to restoration of hemostasis in Group A was 11.4 hours. In Group B, normal intraoperative hemostasis was reported in more than 90% of the patients who underwent procedures after the administration of PRAXBIND®.
The authors concluded that PRAXBIND® was, within minutes, able to completely reverse the anticoagulant effect of PRADAXA®. This reversal agent will fulfill an unmet need and with the availability soon of Factor Xa inhibitor antidotes, it is anticipated that Health Care Providers will be more willing to replace COUMADIN® with the Newer Oral Anticoagulants. Idarucizumab for Dabigatran Reversal. Pollack CV, Reilly PA, Eikelboom J, et al. N Engl J Med 2015; 373:511-520

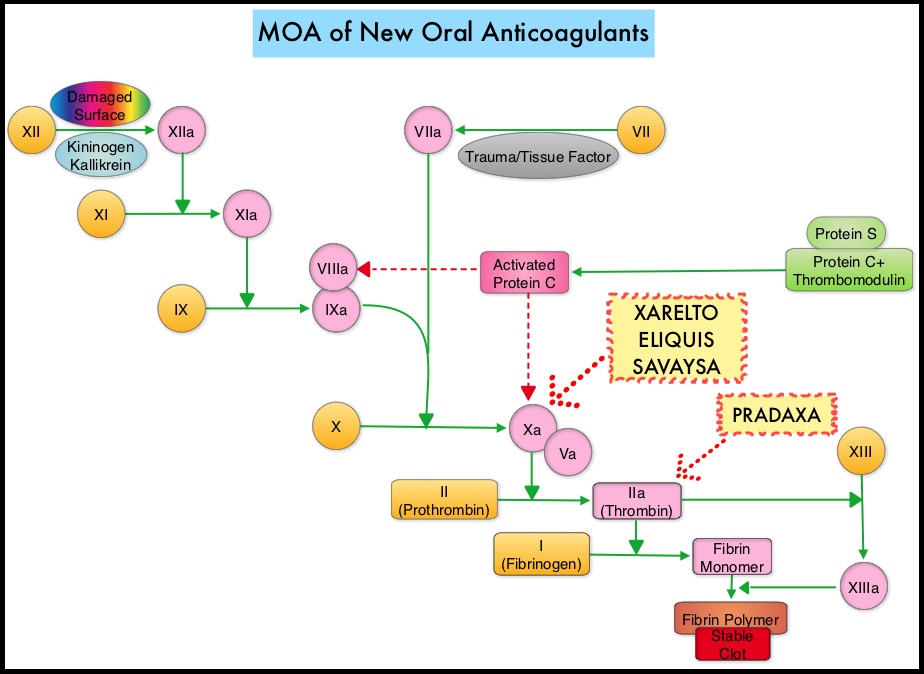 The half life of these agents can however be prolonged in those with renal insufficiency. In several clinical studies, these New Oral Anticoagulants have been shown to reduce the rate of major bleeding by 28% and the rates of intracranial and fatal hemorrhage by 50%, when compared to COUMADIN®. Unlike bleeding caused by COUMADIN®, which can be reversed using Vitamin K or Fresh Frozen Plasma, there are no specific agents presently available, for reversing bleeding caused by the New Oral Anticoagulants or for stopping the anticoagulant effects of these drugs, in patients who need urgent s
The half life of these agents can however be prolonged in those with renal insufficiency. In several clinical studies, these New Oral Anticoagulants have been shown to reduce the rate of major bleeding by 28% and the rates of intracranial and fatal hemorrhage by 50%, when compared to COUMADIN®. Unlike bleeding caused by COUMADIN®, which can be reversed using Vitamin K or Fresh Frozen Plasma, there are no specific agents presently available, for reversing bleeding caused by the New Oral Anticoagulants or for stopping the anticoagulant effects of these drugs, in patients who need urgent s
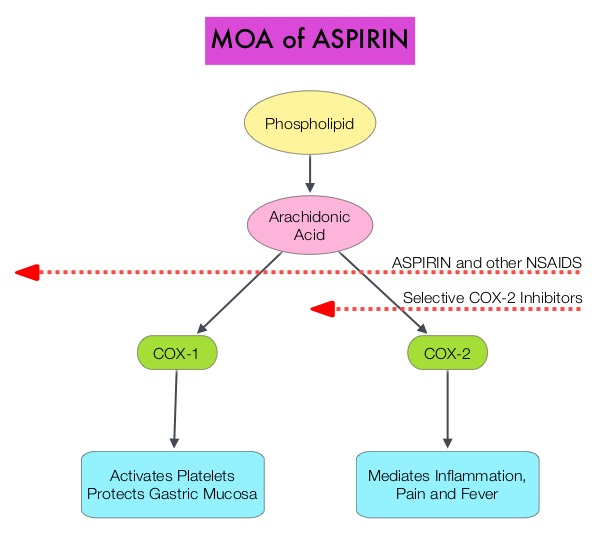 Aspirin is a non-selective, irreversible inhibitor of both COX-1 and COX-2 (Cyclooxygenases 1 and 2). It inhibits the production of Prostaglandins and Thromboxane A2 and there by decreases platelet aggregation. Both ASPIRE and WARFASA trials support the role of aspirin for the prevention of recurrent VTE in patients with an unprovoked VTE, after 6-18 months of anticoagulation therapy. These trials however were not individually powered to detect benefits of treatment for particular outcomes or subgroups of patients. The INSPIRE Collaboration involved further analysis of these trials to detect outcomes in pre-specified subgroups of patients. The intent-to-treat analysis included 1,224 patients and at a median follow up of 30.4 months, daily Aspirin given at a dose of 100mg reduced recurrent VTE events by 32% (HR=0.68; P=0.008) and this included reduction in recurrent Deep Vein Thrombosis by 34% (HR=0.66; P=0.01) and Pulmonary Embolism by 34% (HR=0.66; P=0.08), compared to Placebo. After adjustment for treatment adherence, recurrent VTE was reduced by 42% (HR=0.58; P=0.005). Subgroup analyses indicated similar relative, but larger absolute, risk reductions in men and older patients. Aspirin also reduced major vascular events by 44% (HR=0.66; P=0.002). The major bleeding rate was low at 0.5%/year for Aspirin and 0.4%/year for Placebo. The authors concluded that aspirin reduces the risk of recurrent VTE. Even though the efficacy of aspirin is inferior to that of Warfarin or the new oral anticoagulants, this data suggests that Aspirin should be strongly considered in patients with unprovoked VTE for whom long-term anticoagulation therapy with Warfarin or one of the new oral anticoagulants has to be discontinued or is not an appropriate option. Aspirin however, should not be considered a replacement option for conventional anticoagulation. Simes J, Becattini C, Agnelli G, et al. Circulation. 2014; 130:1062-1071
Aspirin is a non-selective, irreversible inhibitor of both COX-1 and COX-2 (Cyclooxygenases 1 and 2). It inhibits the production of Prostaglandins and Thromboxane A2 and there by decreases platelet aggregation. Both ASPIRE and WARFASA trials support the role of aspirin for the prevention of recurrent VTE in patients with an unprovoked VTE, after 6-18 months of anticoagulation therapy. These trials however were not individually powered to detect benefits of treatment for particular outcomes or subgroups of patients. The INSPIRE Collaboration involved further analysis of these trials to detect outcomes in pre-specified subgroups of patients. The intent-to-treat analysis included 1,224 patients and at a median follow up of 30.4 months, daily Aspirin given at a dose of 100mg reduced recurrent VTE events by 32% (HR=0.68; P=0.008) and this included reduction in recurrent Deep Vein Thrombosis by 34% (HR=0.66; P=0.01) and Pulmonary Embolism by 34% (HR=0.66; P=0.08), compared to Placebo. After adjustment for treatment adherence, recurrent VTE was reduced by 42% (HR=0.58; P=0.005). Subgroup analyses indicated similar relative, but larger absolute, risk reductions in men and older patients. Aspirin also reduced major vascular events by 44% (HR=0.66; P=0.002). The major bleeding rate was low at 0.5%/year for Aspirin and 0.4%/year for Placebo. The authors concluded that aspirin reduces the risk of recurrent VTE. Even though the efficacy of aspirin is inferior to that of Warfarin or the new oral anticoagulants, this data suggests that Aspirin should be strongly considered in patients with unprovoked VTE for whom long-term anticoagulation therapy with Warfarin or one of the new oral anticoagulants has to be discontinued or is not an appropriate option. Aspirin however, should not be considered a replacement option for conventional anticoagulation. Simes J, Becattini C, Agnelli G, et al. Circulation. 2014; 130:1062-1071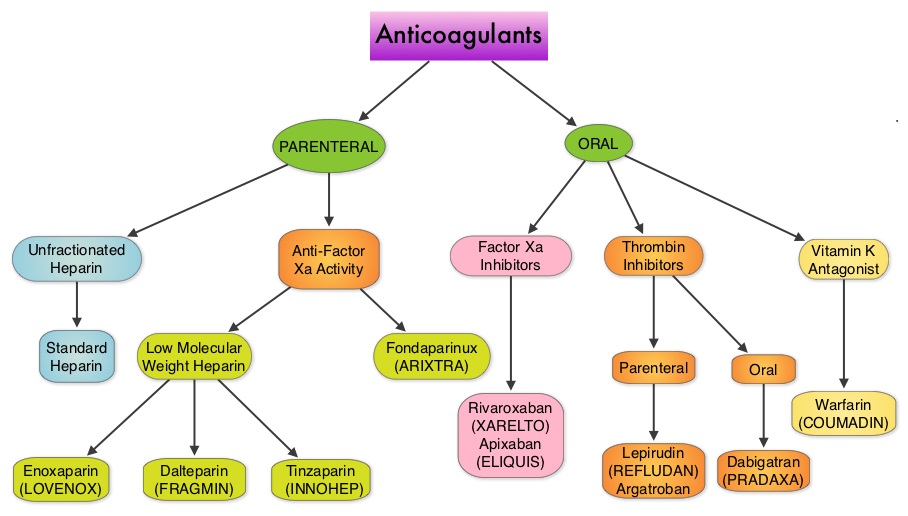 Even though Low Molecular Weight Heparin (LMWH) preparations as well as new oral anticoagulants have been available for the treatment Venous ThromboEmbolism, there has been very little guidance for Health Care Providers on the use of these newer agents. The authors in this analysis compared the efficacy and safety outcomes associated with different anticoagulation regimens for treatment of Venous ThromboEmbolism (VTE). These anticoagulant regimens included Unfractionated Heparin (UFH), Low Molecular Weight Heparin (LMWH) or Fondaparinux in combination with Vitamin K antagonists, LMWH with Dabigatran (PRADAXA®), Rivaroxaban (XARELTO®), Apixaban (ELIQUIS®) or Edoxaban and LMWH alone. This meta analysis included 44,989 patients from 45 randomized trials which reported rates of recurrent VTE and major bleeding in patients with acute VTE. In these Acute Deep Vein Thrombosis and Pulmonary Embolism trials, Rivaroxaban and Apixaban were evaluated without the use of initial LMWH whereas both Dabigatran or Edoxaban were assessed following an initial 5 day treatment with LMWH. This analysis was therefore able to assess clinical and safety outcomes associated with different anticoagulation regimens. The followings findings were noted:
Even though Low Molecular Weight Heparin (LMWH) preparations as well as new oral anticoagulants have been available for the treatment Venous ThromboEmbolism, there has been very little guidance for Health Care Providers on the use of these newer agents. The authors in this analysis compared the efficacy and safety outcomes associated with different anticoagulation regimens for treatment of Venous ThromboEmbolism (VTE). These anticoagulant regimens included Unfractionated Heparin (UFH), Low Molecular Weight Heparin (LMWH) or Fondaparinux in combination with Vitamin K antagonists, LMWH with Dabigatran (PRADAXA®), Rivaroxaban (XARELTO®), Apixaban (ELIQUIS®) or Edoxaban and LMWH alone. This meta analysis included 44,989 patients from 45 randomized trials which reported rates of recurrent VTE and major bleeding in patients with acute VTE. In these Acute Deep Vein Thrombosis and Pulmonary Embolism trials, Rivaroxaban and Apixaban were evaluated without the use of initial LMWH whereas both Dabigatran or Edoxaban were assessed following an initial 5 day treatment with LMWH. This analysis was therefore able to assess clinical and safety outcomes associated with different anticoagulation regimens. The followings findings were noted: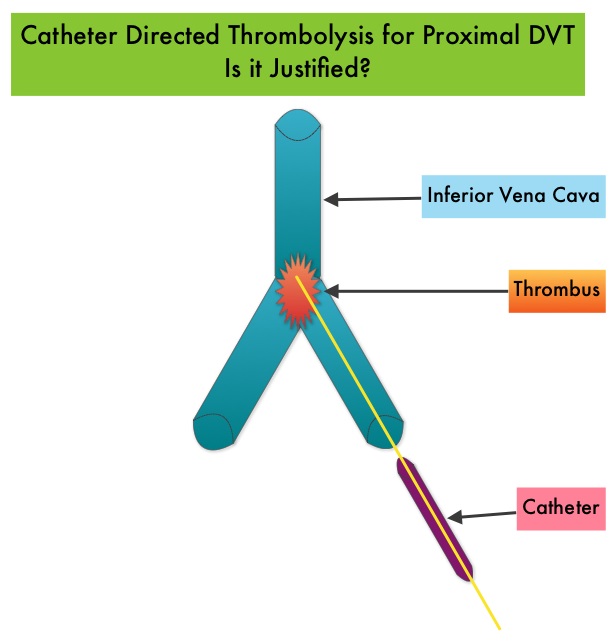 Several small clinical studies have shown a decrease in the incidence of Post Thrombotic Syndrome, with Catheter Directed Thrombolysis (CDT), although safety outcomes from this intervention remained inconclusive. This had resulted in conflicting recommendations from the American Heart Association and American College of Chest Physicians. To address this further, the authors in this study compared safety outcomes in a group of patients with proximal and caval DVT who underwent CDT plus anticoagulation with another group treated with anticoagulation alone. In this propensity-matched analysis, 90,618 patients with a discharge diagnosis of proximal DVT over a 6 year period and 3,594 well-matched patients in each study group were identified from a Nationwide Inpatient Sample (NIS) database. The primary endpoint of this study was in-hospital mortality and secondary endpoints included incidence of pulmonary embolism, blood transfusion requirements, gastrointestinal bleeding, intracranial bleeding, IVC filter placement, procedure-related hematomas, length of hospital stay and hospital charges. This analysis did not demonstrate a significant difference in the in-hospital mortality between patients who had Catheter Directed Thrombolysis (CDT) plus anticoagulation group and the anticoagulation alone group (1.2% vs 0.9%). However, the rates of blood transfusion (11.1% vs 6.5%), pulmonary embolism (17.9% vs 11.4%), intracranial hemorrhage (0.9% vs 0.3%) and IVC filter placement (34.8% vs15.6%), were significantly higher in the CDT group compared to the anticoagulation alone group. Further, patients in the CDT group had a significantly longer hospital stay (7.2 vs. 5.0 days) and incurred significantly higher hospital expenses ($85,094 vs $28,164). More patients with private insurance had Catheter Directed Thrombolysis (CDT), compared to self-pay patients or those with Medicare or Medicaid (7.3% vs 2.8%, P<0.001). The authors concluded that Catheter Directed Thrombolysis (CDT) may not improve outcomes for patients with proximal Deep Vein Thrombosis but is associated with significantly higher adverse events and therefore warrants substantial justification, if this intervention is planned. Bashir R, Zack CJ, Zhao H, et al. JAMA Intern Med. 2014;174:1494-1501
Several small clinical studies have shown a decrease in the incidence of Post Thrombotic Syndrome, with Catheter Directed Thrombolysis (CDT), although safety outcomes from this intervention remained inconclusive. This had resulted in conflicting recommendations from the American Heart Association and American College of Chest Physicians. To address this further, the authors in this study compared safety outcomes in a group of patients with proximal and caval DVT who underwent CDT plus anticoagulation with another group treated with anticoagulation alone. In this propensity-matched analysis, 90,618 patients with a discharge diagnosis of proximal DVT over a 6 year period and 3,594 well-matched patients in each study group were identified from a Nationwide Inpatient Sample (NIS) database. The primary endpoint of this study was in-hospital mortality and secondary endpoints included incidence of pulmonary embolism, blood transfusion requirements, gastrointestinal bleeding, intracranial bleeding, IVC filter placement, procedure-related hematomas, length of hospital stay and hospital charges. This analysis did not demonstrate a significant difference in the in-hospital mortality between patients who had Catheter Directed Thrombolysis (CDT) plus anticoagulation group and the anticoagulation alone group (1.2% vs 0.9%). However, the rates of blood transfusion (11.1% vs 6.5%), pulmonary embolism (17.9% vs 11.4%), intracranial hemorrhage (0.9% vs 0.3%) and IVC filter placement (34.8% vs15.6%), were significantly higher in the CDT group compared to the anticoagulation alone group. Further, patients in the CDT group had a significantly longer hospital stay (7.2 vs. 5.0 days) and incurred significantly higher hospital expenses ($85,094 vs $28,164). More patients with private insurance had Catheter Directed Thrombolysis (CDT), compared to self-pay patients or those with Medicare or Medicaid (7.3% vs 2.8%, P<0.001). The authors concluded that Catheter Directed Thrombolysis (CDT) may not improve outcomes for patients with proximal Deep Vein Thrombosis but is associated with significantly higher adverse events and therefore warrants substantial justification, if this intervention is planned. Bashir R, Zack CJ, Zhao H, et al. JAMA Intern Med. 2014;174:1494-1501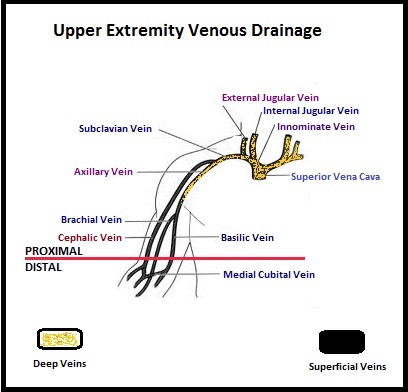 The authors characterized the NLDVT as Prevalent or Incident (depending on whether the thrombosis was identified within 72 hours of ICU admission or developed after the third ICU day) and whether they were catheter related or not. Risk factors for NLDVT and subsequent anticoagulant therapy, associated PE, and death were evaluated. Several important findings were noted from this study. Of the 3746 patients, 2.2% developed 1 or more Non-Leg Vein Thromboses (superficial or deep, proximal or distal). Majority of these thrombotic events (95%) occurred in the upper extremity and most of these occurred in the clinically important proximal and deep venous system. Further, these thrombotic episodes were more commonly Incident (2.0%) rather than Prevalent (0.2%) – P <0.001. It was noted that 1 in 7 patients with NLDVT developed PE. It appears that malignancy, hospitalization and Central Venous Catheters are risk factors for Upper Extremity Deep Vein Thrombosis (UEDVT), but the disproportionate increase in the incidence of UEDVT in hospitalized patients (30%-40% of hospital associated DVT’s) has been attributed to the increased use of CVC’s such as PICC (Peripherally Inserted Central Catheter). In this study, only 13% of the patients diagnosed with NLDVT received anticoagulation therapy. In an accompanying commentary by Dr. Greg Maynard, it was well pointed out that UEDVT is associated with similar rates of recurrence, PE and mortality as lower extremity DVT and as such UEDVT should be treated with anticoagulant therapy similar to Lower Extremity DVT. Dr. Maynard also noted that PICC associated DVT could be significantly reduced by appropriately choosing patients for a PICC line, proper PICC placement, early PICC removal, smaller diameter PICC and smaller number of lumens in the catheter. The authors concluded that despite universal prophylaxis with heparin, there was a high incidence of NLDVT in clinically important locations of the venous system, in critically ill patients and this calls for more structured preventive measures, as we learn more about this entity. Lamontagne F, McIntyre L, Dodek P, et al. JAMA Intern Med. 2014;174:689-696
The authors characterized the NLDVT as Prevalent or Incident (depending on whether the thrombosis was identified within 72 hours of ICU admission or developed after the third ICU day) and whether they were catheter related or not. Risk factors for NLDVT and subsequent anticoagulant therapy, associated PE, and death were evaluated. Several important findings were noted from this study. Of the 3746 patients, 2.2% developed 1 or more Non-Leg Vein Thromboses (superficial or deep, proximal or distal). Majority of these thrombotic events (95%) occurred in the upper extremity and most of these occurred in the clinically important proximal and deep venous system. Further, these thrombotic episodes were more commonly Incident (2.0%) rather than Prevalent (0.2%) – P <0.001. It was noted that 1 in 7 patients with NLDVT developed PE. It appears that malignancy, hospitalization and Central Venous Catheters are risk factors for Upper Extremity Deep Vein Thrombosis (UEDVT), but the disproportionate increase in the incidence of UEDVT in hospitalized patients (30%-40% of hospital associated DVT’s) has been attributed to the increased use of CVC’s such as PICC (Peripherally Inserted Central Catheter). In this study, only 13% of the patients diagnosed with NLDVT received anticoagulation therapy. In an accompanying commentary by Dr. Greg Maynard, it was well pointed out that UEDVT is associated with similar rates of recurrence, PE and mortality as lower extremity DVT and as such UEDVT should be treated with anticoagulant therapy similar to Lower Extremity DVT. Dr. Maynard also noted that PICC associated DVT could be significantly reduced by appropriately choosing patients for a PICC line, proper PICC placement, early PICC removal, smaller diameter PICC and smaller number of lumens in the catheter. The authors concluded that despite universal prophylaxis with heparin, there was a high incidence of NLDVT in clinically important locations of the venous system, in critically ill patients and this calls for more structured preventive measures, as we learn more about this entity. Lamontagne F, McIntyre L, Dodek P, et al. JAMA Intern Med. 2014;174:689-696