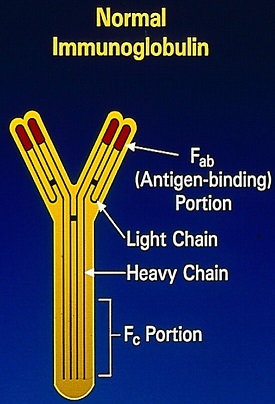
SUMMARY: The FDA on November 6, 2018 approved EMPLICITI® (Elotuzumab) in combination with POMALYST® (Pomalidomide) and Dexamethasone for the treatment of adult patients with Multiple Myeloma who have received at least two prior therapies, including REVLIMID® (Lenalidomide) and a Proteasome Inhibitor. Multiple Myeloma is a clonal disorder of plasma cells in the bone marrow and the American Cancer Society estimates that in the United States, 30,770 new cases will be diagnosed in 2018 and 12,770 patients are expected to die of the disease. Multiple Myeloma (MM) in 2018 remains an incurable disease. The therapeutic goal therefore is to improve Progression Free Survival (PFS) and Overall Survival (OS). Despite the introduction of novel therapies for the treatment of Multiple Myeloma, many patients still face poor outcomes in the Relapsed/Refractory setting.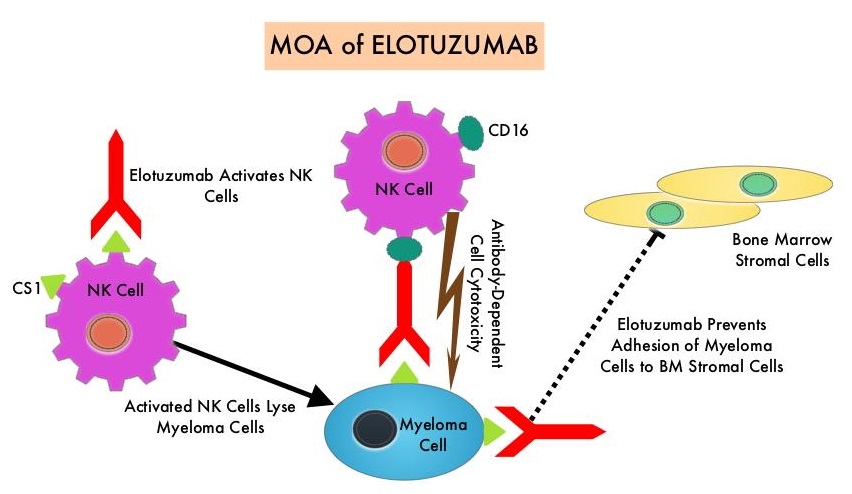
EMPLICITI® is a immunostimulatory monoclonal antibody that binds to the Signal Lymphocyte Activation Molecule – SLAMF7 protein (CS1, CD319), which is highly expressed on Myeloma cells and also expressed on Natural Killer (NK) lymphocytes in the immune system. By virtue of its dual mechanism of action, it targets and destroys Myeloma cells and also enhances the activation of Natural Killer cells. The FDA in November 2015, approved EMPLICITI® for use in combination with REVLIMID® and Dexamethasone for the treatment of patients with Multiple Myeloma who received one to three prior therapies, based on the ELOQUENT-2 trial.
ELOQUENT-3 is a multicenter, randomized, open label, phase II trial in which 117 patients with Relapsed/Refractory Multiple Myeloma were randomly assigned, in a 1:1 ratio, to receive EMPLICITI® plus POMALYST® and Dexamethasone (N=60) – EMPLICITI® group or POMALYST® and Dexamethasone (N= 57) – control group. Treatment was administered in 28-day cycles. Patients in the EMPLICITI® group received EMPLICITI® 10 mg/kg IV days 1, 8, 15, and 22 during cycles 1 and 2 and 20 mg/kg on day 1 of each cycle thereafter. POMALYST® was given at 4 mg orally on days 1 thru 21 of each cycle along with Dexamethasone 40 mg weekly for patients 75 years of age or less or 20 mg for those more than 75 years of age. Treatment was continued until disease progression or unacceptable toxicity. Eligible patients had received 2 or more prior lines of therapy and prophylaxis against thromboembolism was required for all patients. The median age of patients was 67 years. Prior treatments included VELCADE®- Bortezomib (100%), REVLIMID® (99%), KYPROLIS® – Carfilzomib (21%), NINLARO® – Ixazomib (6%), and DARZALEX® – Daratumumab (3%). Close to 55% of patients had undergone Stem Cell transplantation and most patients were refractory to REVLIMID® (87%), a Proteosome Inhibitor (80%), or both (70%). The Primary end point was Progression Free Survival (PFS). The Secondary endpoints included Overall Response Rate (ORR), Complete Response (CR), Stringent Complete Response, Very Good Partial Response and Partial Response Rates.
After a minimum follow up period of 9.1 months, the median PFS was 10.3 months in the EMPLICITI® group and 4.7 months in the control group (HR=0.54; P=0.008), which suggested a 46% reduction in the risk of disease progression. The PFS of EMPLICITI® was consistently observed across all predefined patient subgroups. The Overall Response Rate was 53% in the EMPLICITI® group as compared with 26% in the control group, suggesting a doubling in the Response Rates in the EMPLICITI® group (P=0.0029) with Very Good Partial Responses or better seen in 20% of those in the EMPLICITI® group. The Overall Survival data were immature at the time of the analysis. However, a trend favoring the EMPLICITI® group was observed (HR for death=0.62).
The incidence of serious adverse events was 53% in the EMPLICITI® group and 55% in the control group and the most common treatment-related adverse events in the EMPLICITI® and control groups were neutropenia (18% versus 20%), hyperglycemia (18% versus 11%) and anemia (10% versus 15%), respectively. Adverse events that led to discontinuation of treatment occurred in 18% of the patients in the EMPLICITI® group and in 24% of the patients in the control group.
It was concluded that among patients with Multiple Myeloma who had progressed on REVLIMID® and a Proteosome Inhibitor, EMPLICITI® combined with POMALYST® and Dexamethasone significantly decreased the risk of progression or death and also doubled the Response Rate, compared to POMALYST® plus Dexamethasone alone. Elotuzumab plus Pomalidomide and Dexamethasone for Multiple Myeloma. Dimopoulos MA, Dytfeld D, Grosicki S, et al. N Engl J Med 2018;379:1811-1822