The FDA on November 21, 2016 approved DARZALEX® in combination with REVLIMID® (Lenalidomide) and Dexamethasone, or VELCADE® (Bortezomib) and Dexamethasone, for the treatment of patients with multiple myeloma, who have received at least one prior therapy. DARZALEX® is a product of Janssen Biotech, Inc.
Tag: Multiple Myeloma
Deep Clinical Responses and MRD Negativity with DARZALEX® in Multiple Myeloma
SUMMARY: Multiple Myeloma is a clonal disorder of plasma cells in the bone marrow and the American Cancer Society estimates that in the United States, about 30,280 new cases will be diagnosed in 2017 and 12,590 patients will die of the disease. Multiple Myeloma is a disease of the elderly, with a median age at diagnosis of 69 years and characterized by intrinsic clonal heterogeneity. With a record number of regulatory approvals for Myeloma treatment over the past 12 years, the median survival for patients with Myeloma is over 10 years. The recent new drugs approved for the treatment of relapsed/refractory Multiple Myeloma include a Histone Decetylase inhibitor (FARYDAK®) and 2 monoclonal antibodies, Daratumumab (DARZALEX®) and Elotuzumab (EMPLICITI®).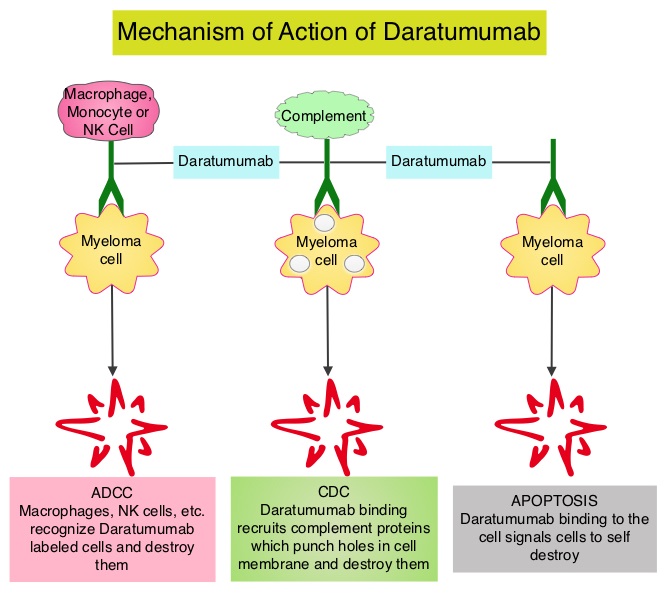
Testing for MRD (Minimal Residual Disease) is standard practice in hematological malignancies such as Chronic Myeloid Leukemia (CML) which is characterized by a defined genetic abnormality ie. Philadelphia Chromosome, and CML also has very effective therapies. Unlike CML, multiple myeloma is a heterogeneous disorder and very effective therapies are only now becoming available. Effective combination regimens for myeloma has resulted in high response rates and therefore achievement of MRD negativity may be a primary endpoint in the near future. MRD negativity has been associated with prolonged Progression Free Survival (PFS) and Overall Survival (OS), in several meta-analyses.
Daratumumab (DARZALEX®) is a human IgG1 antibody that targets CD38, a transmembrane glycoprotein abundantly expressed on malignant plasma cells and with low levels of expression on normal lymphoid and myeloid cells. DARZALEX® exerts its cytotoxic effect on myeloma cells by multiple mechanisms, including Antibody Dependent Cellular Cytotoxicity (ADCC), Complement Mediated Cytotoxicity and direct apoptosis. Additionally, DARZALEX® may have a role in immunomodulation by depleting CD38-positive regulator Immune suppressor cells, and thereby expanding T cells, in patients responding to therapy.
The authors in this publication prospectively evaluated Minimal Residual Disease (MRD) status of patients enrolled in two large phase III trials, the POLLUX and CASTOR studies, and assessed the ability of DARZALEX® to yield deep clinical responses beyond complete remission. In the POLLUX study, 569 patients with relapsed or refractory multiple myeloma were randomized in a 1:1 ratio to receive either DARZALEX®, REVLIMID® (Lenalidomide) and Dexamethasone or REVLIMID® and Dexamethasone. In the CASTOR study, 498 patients with relapsed or refractory multiple myeloma were randomized in a 1:1 ratio to receive either DARZALEX®, VELCADE® (Bortezomib) and Dexamethasone or VELCADE® and Dexamethasone. In both these studies, the addition of DARZALEX® resulted in significant improvements in median PFS (HR=0.37; P<0.001 in the POLLUX study and HR=0.39; P<0.0001) in the CASTOR study), compared to the control group.
The researchers in this study, assessed MRD of bone marrow aspirate samples using ClonoSEQ next-generation sequencing–based assay. In the POLLUX study, MRD was assessed at the time of suspected Complete Response (CR), and at 3 and 6 months after. In the CASTOR study, MRD was assessed at the time of suspected CR, and at 6 months and 12 months after the first dose. The MRD sensitivity thresholds were 0.01% (1 cancer cell per 10,000 nucleated cells, or 10-4), 0.001% (10-5), and 0.0001% (10-6). The MRD negativity rate was defined as the proportion of patients with negative MRD results at any point during the studies. The median follow-up was 13.5 months for the POLLUX study and 7.4 months for the CASTOR study.
In the POLLUX study, the addition of DARZALEX® to REVLIMID® and Dexamethasone improved the MRD-negative status rates from 8.8% to 31.8% at the 10-4 threshold, from 5.7% to 24.8% at the 10-5 threshold, and from 2.5% to 11.9% at the 10-6 threshold. In the CASTOR study, the addition of DARZALEX® to VELCADE® and Dexamethasone improved the MRD-negative status rates from 3.6% to 18.3% at the 10-4 threshold, from 2.4% to 10.4% at the 10-5 threshold, and from 0.8% to 4.4% at the 10-6 threshold. It was noted that the MRD negativity was consistently higher in patients treated with DARZALEX® and DARZALEX® induced MRD negativity in three times as many patients as those treated with standard regimens. Further, MRD negativity was noted as soon as 3 months, with many patients continuing to achieve MRD negativity over time. High risk patients {t(4;14), t(14;16), del17p} benefited as well, with 18% of high risk patients in the POLLUX study and 14% of those in the CASTOR study achieving MRD negativity. Patients with sustained MRD negativity following treatment with DARZALEX®, had a significantly longer PFS compared with the control group.
It was concluded that the addition of DARZALEX®, in relapsed/refractory multiple myeloma, to standard treatment regimens, induced MRD negativity in three times as many patients as those treated with standard regimens. Evaluation of minimal residual disease (MRD) in relapsed/refractory multiple myeloma (RRMM) patients treated with daratumumab in combination with lenalidomide plus dexamethasone or bortezomib plus dexamethasone. Avet-Loisseau H, Casneuf T, Chiu C, et al. Presented at: American Society of Hematology 58th Annual Meeting; December 3-6, 2016; San Diego, CA. Abstract 246
Superior Outcomes with REVLIMID® and Dexamethasone in Elderly Patients with Multiple Myeloma
SUMMARY: Multiple Myeloma is a clonal disorder of plasma cells in the bone marrow and the American Cancer Society estimates that in the United States, about 30,330 new cases will be diagnosed in 2016 and 12,650 patients will die of the disease. Multiple Myeloma is a disease of the elderly, with a median age at diagnosis of 69 years and characterized by intrinsic clonal heterogeneity. Nonetheless, patients 75 years or older, are often under-represented in clinical trials because of increased comorbidities and altered pharmacodynamics. This group of elderly, newly diagnosed Multiple Myeloma patients, who are ineligible for Stem Cell Transplantation (SCT), are often treated with combination therapies such as Melphalan, Prednisone, and Thalidomide (MPT), VELCADE® (Bortezomib), Melphalan, and Prednisone (VMP) or REVLIMID® (Lenalidomide) and low dose Dexamethasone (Rd). However there is limited data regarding the efficacy and safety of front line use of REVLIMID® in this patient group.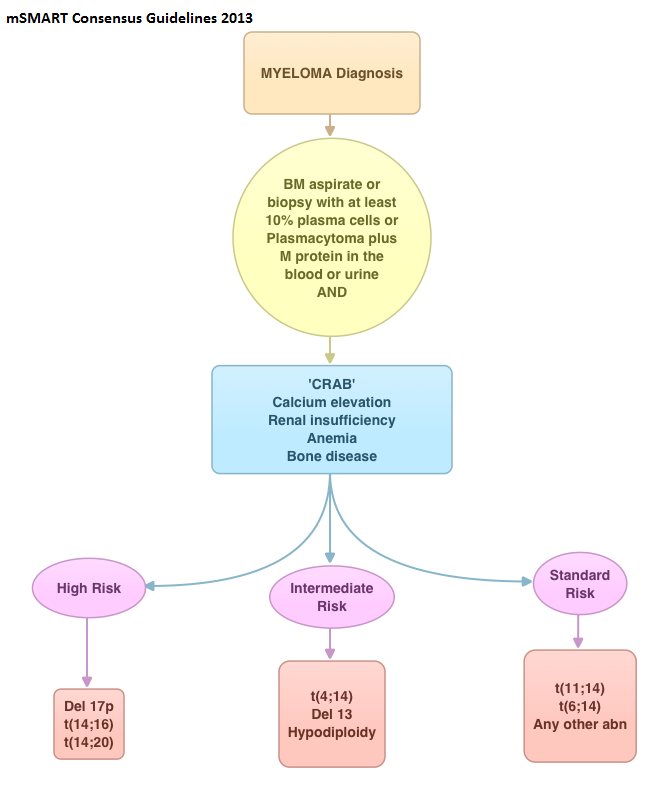
The FIRST (Frontline Investigation of REVLIMID® Plus Dexamethasone Versus Standard Thalidomide) trial is a randomized, global, phase III trial, in which the efficacy and safety of REVLIMID® and Dexamethasone, given until disease progression or for a fixed number of cycles, was compared with MPT given for a fixed number of cycles, in patients with newly diagnosed Multiple Myeloma, who were ineligible for Stem Cell Transplantation. A total of 1,623 patients were randomly assigned patients in a 1:1:1 ratio to receive REVLIMID® and Dexamethasone (Rd continuous) in 28 day cycles until disease progression, REVLIMID® and Dexamethasone (Rd18) in 28 day cycles for 72 weeks (18 cycles), or MPT in 42 day cycles for 72 weeks (12 cycles). In both REVLIMID® groups, patients received REVLIMID® 25 mg orally daily on days 1 – 21 of each 28 day cycle, and Dexamethasone 40 mg orally on days 1, 8, 15, and 22. Patients in the MPT group received Melphalan 0.25 mg/kg/day orally on days 1- 4, Prednisone 2 mg/kg/day orally on days 1- 4 and Thalidomide 200 mg orally daily, administered in 42 day cycles. The median patient age was 73 years and 35% of the patients were older than 75 years. Patients were stratified by age (75 years or less versus more than 75 years) and disease stage. The Primary end point was Progression Free Survival (PFS) and Secondary end point included Overall Survival (OS), Overall Response Rate (ORR), Duration of Response (DOR), time to first response and Time to Treatment Failure. The primary objective was to compare the efficacy of Rd continuous with MPT.
In this updated analysis after a median follow up of 45.5 months, Rd continuous reduced the risk of progression or death by 31% compared with MPT (HR=0.69; P<0.001) overall. In patients 75 years or younger, Rd continuous reduced the risk of progression or death by 36% (HR=0.64; P<0.001) and by 20% (HR=0.80; P=0.08) in those older than 75 years. Patients in the Rd continuous group also had a longer median Overall Survival than those in the MPT group regardless of age, and there was a 14 month Overall Survival advantage for the group of patients 75 years or older. In the Rd continuous group, grade 3-4 adverse events were similar across age groups although older patients had more frequent REVLIMID® dose reductions.
The authors concluded that the FIRST study results, with the largest cohort of patients 75 years or older, support Rd continuous treatment as a new standard of care for Stem Cell Transplantation-ineligible patients with newly diagnosed Multiple Myeloma. Updated Outcomes and Impact of Age With Lenalidomide and Low-Dose Dexamethasone or Melphalan, Prednisone, and Thalidomide in the Randomized, Phase III FIRST Trial Hulin C, Belch A, Shustik C, et al. J Clin Oncol 2016;34:3609-3617
DARZALEX® Combination Demonstrates Impressive Efficacy in Relapsed or Refractory Myeloma
SUMMARY: Multiple Myeloma is a clonal disorder of plasma cells in the bone marrow and the American Cancer Society estimates that in the United States, about 30,330 new cases will be diagnosed in 2016 and 12,650 patients will die of the disease. Multiple Myeloma is a disease of the elderly, with a median age at diagnosis of 69 years and characterized by intrinsic clonal heterogeneity. With a record number of regulatory approvals for Myeloma treatment over the past 12 years, the median survival for patients with Myeloma is over 10 years. The recent new drugs approved for the treatment of relapsed/refractory Multiple Myeloma include a Histone Decetylase inhibitor (FARYDAK®) and 2 monoclonal antibodies, Daratumumab (DARZALEX®) and Elotuzumab (EMPLICITI®).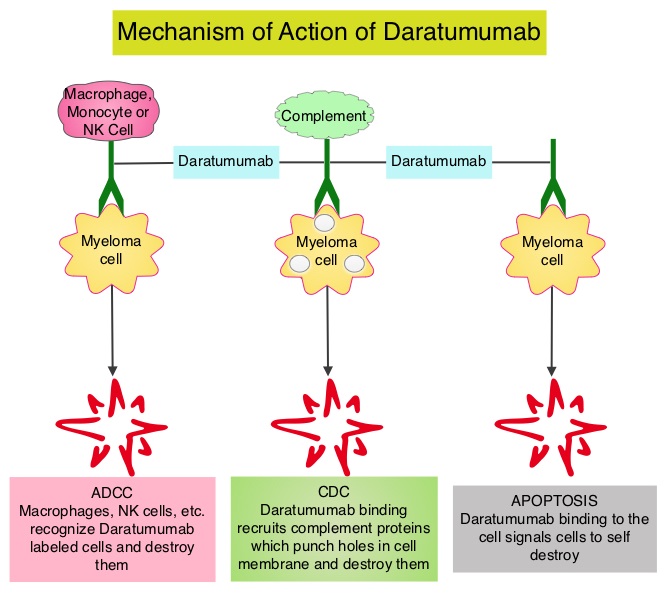
Daratumumab (DARZALEX®) is a human IgG1 antibody that targets CD38, a transmembrane glycoprotein abundantly expressed on malignant plasma cells and with low levels of expression on normal lymphoid and myeloid cells. DARZALEX® exerts its cytotoxic effect on myeloma cells by multiple mechanisms, including Antibody Dependent Cellular Cytotoxicity (ADCC), Complement Mediated Cytotoxicity and direct apoptosis. Additionally, DARZALEX® may have a role in immunomodulation by depleting CD38-positive regulator Immune suppressor cells, and thereby expanding T cells, in patients responding to therapy. Previously published phase I and II studies involving patients with relapsed or refractory multiple myeloma demonstrated promising efficacy of DARZALEX® when given as a single agent, as well as when given along with Lenalidomide (REVLIMID®) and Dexamethasone.
The authors now report the results of a prespecified interim analysis of a phase III trial of DARZALEX®, REVLIMID® and Dexamethasone in patients with relapsed or refractory multiple myeloma. In this randomized, open-label, multicenter, phase III study, 569 patients who had relapsed or refractory multiple myeloma were assigned in a 1:1 ratio to receive either DARZALEX®, REVLIMID® and Dexamethasone (Daratumumab group, N=286) or REVLIMID® and Dexamethasone (Control group, N=283). Patients refractory to REVLIMID® were excluded. Patients in the Daratumumab group received DARZALEX® 16 mg/kg IV on days 1, 8, 15, and 22 of a 28 day cycle for 8 weeks during cycles 1 and 2, every 2 weeks (on days 1 and 15) for 16 weeks (cycles 3 thru 6), and every 4 weeks thereafter. Both treatment groups received REVLIMID® 25 mg PO on days 1-21 of each cycle and Dexamethasone 40 mg PO weekly. The primary end point was Progression Free Survival (PFS). Secondary end points included the time to disease progression, Response Rate, time to response, duration of response, Overall Survival (OS) and percentage of patients with results below the threshold for Minimal Residual Disease. Minimal Residual Disease status was evaluated for patients who had a Complete Response by Next-Generation sequencing assay of bone marrow.
At a median follow-up of 13.5 months, the PFS at 12 months was 83.2% in the Daratumumab group compared to 60.1% in the control group (HR=0.37; P<0.001). The Overall Response Rate was significantly higher in the Daratumumab group than in the control group (92.9% versus 76.4%, P<0.001) and further, there was a higher rate of Complete Response or better (43.1% vs. 19.2%, P<0.001). In the Daratumumab group, 22.4% of the patients had results below the threshold for Minimal Residual Disease (1 tumor cell per 105 white cells), as compared with 4.6% of those in the control group (P<0.001), and those with results below the threshold for Minimal Residual Disease had improved outcomes. The most common grade 3 or 4 adverse events were cytopenias. Approximately 48% of the patients in the Daratumumab group experienced grade 1 or 2 infusion-related reactions.
The authors concluded that the addition of DARZALEX® to REVLIMID® and Dexamathasone significantly improves Progression Free Survival among patients with relapsed or refractory multiple myeloma. This impressive efficacy data may warrant the use of this triplet combination for first relapse, in this group of patients, provided their disease is not refractory to REVLIMID®. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. Dimopoulos MA, Oriol A, Nahi H, et al. for the POLLUX Investigators. N Engl J Med 2016;375:1319-1331
New NCCN Guidelines for the Diagnosis and Treatment of Multiple Myeloma
SUMMARY: Multiple Myeloma is a clonal disorder of plasma cells in the bone marrow and the American Cancer Society estimates that in the United States, about 30,330 new cases will be diagnosed in 2016 and 12,650 patients will die of the disease. Multiple Myeloma is a disease of the elderly, with a median age at diagnosis of 69 years and characterized by intrinsic clonal heterogeneity. With a record number of regulatory approvals for Myeloma treatment over the past 12 years, the median survival for patients with Myeloma is over 10 years. 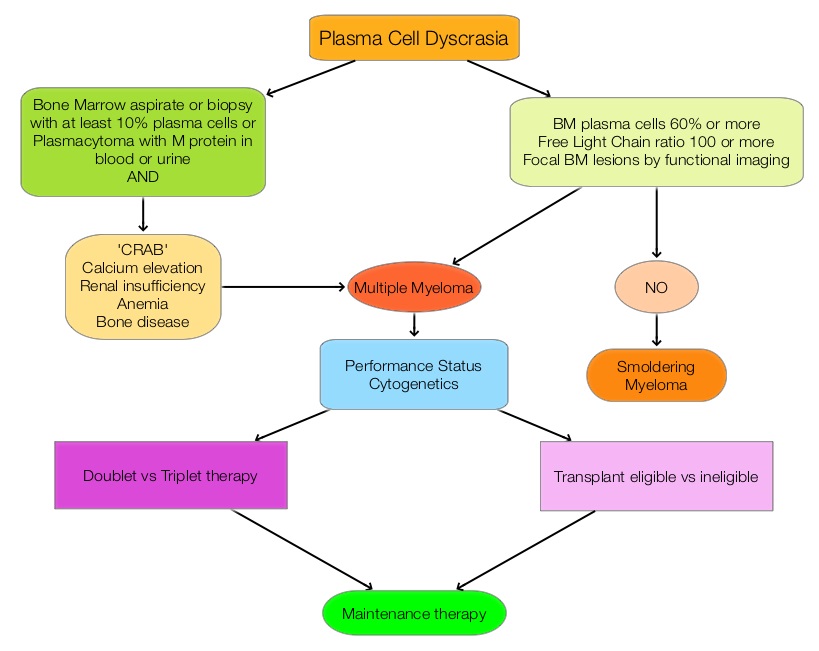 The recent new drugs approved for the treatment of relapsed/refractory Multiple Myeloma include a Histone Decetylase inhibitor (FARYDAK®) and 2 monoclonal antibodies, Daratumumab (DARZALEX®) and Elotuzumab (EMPLICITI®). The two most important determinants of Myeloma patient outcomes include, Performance Status and tumor genomics. Performance Status can be assessed using currently validated instruments and patients can be defined as Fit, Intermediate Fit, or Frail. Patients with chromosomal abnormalities t(4;14), t(14;16), t(14;20) or del 17p are considered to fall in the high risk group.
The recent new drugs approved for the treatment of relapsed/refractory Multiple Myeloma include a Histone Decetylase inhibitor (FARYDAK®) and 2 monoclonal antibodies, Daratumumab (DARZALEX®) and Elotuzumab (EMPLICITI®). The two most important determinants of Myeloma patient outcomes include, Performance Status and tumor genomics. Performance Status can be assessed using currently validated instruments and patients can be defined as Fit, Intermediate Fit, or Frail. Patients with chromosomal abnormalities t(4;14), t(14;16), t(14;20) or del 17p are considered to fall in the high risk group.
The updated Multiple Myeloma guidelines were presented at the National Comprehensive Cancer Network (NCCN) 21st Annual Conference on April 1, 2016. These guidelines include the use of the Revised International Staging System for Multiple Myeloma, treatment of asymptomatic patients even if they do not fit the CRAB criteria (Calcium elevation, Renal insufficiency, Anemia, or Bone abnormalities) and incorporation of a triplet instead of doublet for newly diagnosed Fit Myeloma patients, regardless of transplant eligibility.
Revised International Staging System for Multiple Myeloma
The revised ISS (R-ISS) combines the International Staging System (ISS) with chromosomal abnormalities detected by interphase Fluorescent In Situ Hybridization after CD138 plasma cell purification and serum Lactate DeHydrogenase (LDH).
R-ISS I: ISS stage I (serum β2-microglobulin level less than 3.5 mg/L and serum albumin level 3.5 g/dL or more), absence of high risk cytogenetics and normal LDH level (less than the upper limit of normal range)
R-ISS III: ISS stage III (serum β2-microglobulin level more than 5.5 mg/L) and high-risk cytogenetics or high LDH level
R-ISS II: Includes all the other possible combinations
New Definition of Active MM in Asymptomatic Patients Qualifying for Treatment
• Bone marrow plasmacytosis 60% or more
• Abnormal free light chain ratio 100 or more (involved kappa) or less than 0.01 (involved lambda)
• Focal bone marrow lesions detected by functional imaging such as a PET scan or a MRI
New Treatment Options
For newly diagnosed patients, who are transplant candidates or Fit non-transplant candidates, Lenalidomide/Bortezomib/Dexamethasone should be incorporated as the preferred regimen based on a phase III randomized trial showing superiority of this triplet therapy over a doublet. This triplet improved Complete Response (CR) rates (including molecular CR), Progression Free Survival (PFS), and Overall Survival (OS) compared with Lenalidomide/Dexamethasone doublet. (Blood. 2015;126:25). Another all oral triplet included in the guidelines is a combination of Lenalidomide, Ixazomib and Dexamethasone. Ixazomib (NINLARO®) is an oral proteasome inhibitor and has a half-life of 3 to 4 days and requires only once weekly administration. The combination of Carfilzomib/Lenalidomide, and Dexamethasone was included in the new guidelines as a category 2A front-line treatment, based on a phase 1/2 study (Blood. 2012;120:1801-1809). Maintenance therapy is now considered standard of care regardless of transplantation and for newly diagnosed non-transplant candidates, a new standard of care is continuous Lenalidomide/Dexamethasone, which was found to improve PFS significantly, with a favorable safety profile (N Engl J Med. 2014;371:906-917). Three drug maintenance therapy may benefit high risk patients and should be considered.
In conclusion, these latest updates reflect the rapid advances in the diagnosis and treatment of Multiple Myeloma. National Comprehensive Cancer Network (NCCN) 21st Annual Conference. Presented April 1, 2016, Hollywood, FL.
EMPLICITI® (Elotuzumab)
The FDA on November 30, 2015 approved EMPLICITI® in combination with Lenalidomide and Dexamethasone for the treatment of patients with Multiple Myeloma who have received one to three prior therapies. EMPLICITI® is a product of Bristol-Myers Squibb Company.
First Oral Triplet Combination – NINLARO®, REVLIMID® and Dexamethasone for Multiple Myeloma
SUMMARY: Multiple Myeloma is a clonal disorder of plasma cells in the bone marrow and the American Cancer Society estimates that in the United States, about 30,330 new cases will be diagnosed in 2016 and 12,650 patients will die of the disease. Proteasomes are enzymes found in cells and they enable the breakdown of abnormal or mutant proteins. The amino acids from these proteins are recycled to make new proteins. Myeloma cells depend on the proteasomes to facilitate this metabolic function, to regulate their growth and survival. NINLARO® (Ixazomib) unlike VELCADE® (Bortezomib) is a second generation, oral, proteasome inhibitor, which disrupts protein metabolism in Myeloma cells, by inhibiting proteasomes and has an antiproliferative and pro-apoptotic effect.
The approval of NINLARO® was based a pivotal, multicenter, randomized, double-blind, placebo-controlled, phase III trial (TOURMALINE-MM1 study), in which 722 patients with Multiple Myeloma were randomized in a 1:1 ratio to receive either a combination of NINLARO®, REVLIMID® and Dexamethasone (N=360) or a combination of Placebo, REVLIMID® and Dexamethasone (n=362). NINLARO® was administered at 4 mg PO on days 1, 8, and 15 in combination with REVLIMID® 25 mg PO on days 1 thru 21 and Dexamethasone 40 mg PO on days 1, 8, 15, and 22 of a 28 day treatment cycle. Treatment was continued until disease progression or unacceptable toxicity. Enrolled patients had received 1 to 3 prior lines of therapy, which included VELCADE® (69%), THALOMID® (45%), and REVLIMID® (12%) and 77% of the patients had relapsed Multiple Myeloma. The median age of patients was 66 years. The primary end point of the study was Progression Free survival (PFS) and secondary endpoints included Objective Response Rate (ORR), safety, and Overall Survival.
At a median follow-up of 14.7 months, the PFS with the combination of NINLARO®, REVLIMID® and Dexamethasone was 20.6 months compared with 14.7 months for the combination group of Placebo, REVLIMID® and Dexamethasone (HR= 0.74, P=0.01). This benefit in the NINLARO® group, was noted in all prespecified patient subgroups, including those with high risk cytogenetic abnormalities. The Objective Response Rate was 78% in the NINLARO® group and 72% in the placebo group, and the Complete Response plus Very Good Partial Response in these two treatment groups were 48% and 39% respectively. The median time to response was 1.1 months in the NINLARO® group and 1.9 months in the placebo group and the median duration of response was 20.5 months and 15.0 months respectively. At a median follow up of 23 months, the Overall Survival has not been reached in either study group. Serious adverse events (at least grade 3) were similar in the two study groups (47% in the NINLARO® group and 49% in the placebo group). Patients in the NINLARO® group experienced more adverse events such as thrombocytopenia, vomiting, diarrhea, peripheral neuropathy and skin rash. However, patient-reported Quality of Life was similar in both treatment groups.
The authors concluded that NINLARO® based oral triplet therapy significantly prolonged Progression Free Survival compared with REVLIMID® and Dexamethasone, in patients with relapsed/refractory Multiple Myeloma, with acceptable toxicities. Studies are underway, evaluating NINLARO® in newly diagnosed Myeloma patients as well as maintenance therapy in non-transplant patients. Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. Moreau P, Masszi T, Grzasko N, et al. N Engl J Med 2016; 374:1621-1634
NINLARO® (Ixazomib)
The FDA on November 20, 2015 approved NINLARO® in combination with Lenalidomide and Dexamethasone for the treatment of patients with multiple myeloma who have received at least one prior therapy. NINLARO® is the first approved oral proteasome inhibitor and is a product of Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
DARZALEX® (Daratumumab)
The FDA on November 16, 2015 granted accelerated approval to DARZALEX®, administered as a single agent, for the treatment of patients with multiple myeloma who have received at least three prior lines of therapy, including a Proteasome Inhibitor (PI) and an immunomodulatory agent, or who are double-refractory to a PI and an immunomodulatory agent. DARZALEX® is a product of Janssen Biotech, Inc.
Continuous Therapy Significantly Improves Outcomes compared to Fixed Duration of Therapy in Patients with Newly Diagnosed Multiple Myeloma
SUMMARY: Multiple Myeloma is a clonal disorder of plasma cells in the bone marrow and the American Cancer Society estimates that in the United States, close to 27,000 new cases will be diagnosed in 2015 and 11,240 will die of the disease. Maintenance or Continuous Treatment in patients with newly diagnosed multiple myeloma following induction and consolidation, can result in significantly longer Progression Free Survival (PFS) and Overall Survival (OS), compared to those patients who receive therapy for a fixed duration of time. Not all studies however, have shown Overall Survival benefit. It has been hypothesized that Continuous Treatment could result in resistance to therapy which in turn could reduce the duration of subsequent remission after first relapse and negatively impact overall survival.To address this controversy, the authors conducted a pooled analysis of the outcomes of three randomized phase III trials, coordinated by the same principal investigator, designed to compare Continuous Treatment to Fixed Duration Therapy, in patients with newly diagnosed multiple myeloma.
In trial RV-MM-209, patients were randomized to either induction with Lenolidomide (REVLIMID®), followed by consolidation and subsequent maintenance with REVLIMID® (Continuous Treatment) or Fixed Duration Therapy which entailed REVLIMID® based induction followed by consolidation but no maintenance therapy. In the GIMEMA0305 trial, the randomization was between Bortezomib (VELCADE®) based induction followed by maintenance treatment (Continuous Treatment) and VELCADE® induction, with no maintenance treatment (Fixed Duration Therapy). In the CC-5013-MM-015 study, the comparison was between REVLIMID® in combination with Melphalan and Prednisone followed by REVLIMID® maintenance until disease progression and placebo given along with Melphalan and Prednisone.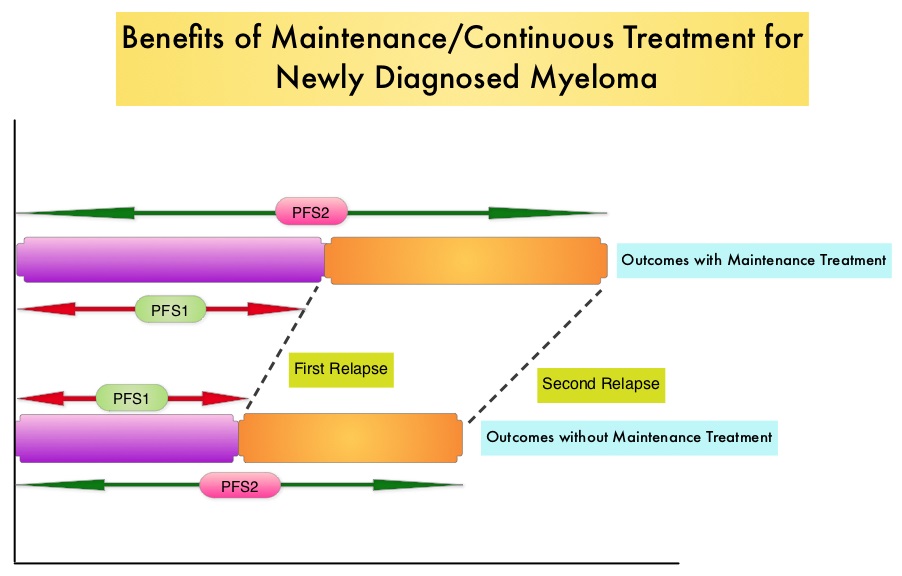
The trial investigators assessed PFS1 as the time from diagnosis to the occurrence of 1st relapse, PFS2 as time from diagnosis to the occurrence of 2nd relapse and Overall Survival as time from diagnosis to death , incorporating the duration of both 1st and 2nd remission. They then evaluated PFS1, PFS2 and OS, in newly diagnosed multiple myeloma patients who received Continuous Therapy or Fixed Duration Therapy. In this pooled analysis of three trials, 604 patients were randomized to Continuous Treatment and 614 patients were randomized to Fixed Duration Therapy. Four hundred and seventeen (N=417) in the Continuous Therapy group and 410 patients in the Fixed Duration Therapy group were eligible for comparative analysis. The median follow up was 52 months.
Patients receiving Continuous Treatment had significantly prolonged PFS1 (median 32 months versus 16 months; HR=0.47; P<0.001), PFS2 (median 55 months versus 40 months; HR=0.61; P=0.001) and OS (4 year OS 69% versus 60%; HR=0.69; P=0.003), when compared with Fixed Dose Therapy. The authors evaluated the PFS and OS from first relapse to second relapse and from first relapse to death respectively, and they noted that the outcomes were similar among patients who received Continuous Treatment or Fixed Dose Therapy following initial diagnosis.
The authors concluded that Continuous Treatment significantly improved PFS1, PFS2, and OS and findings from this pooled analysis suggested that the clinical benefit observed during first remission was not negated by a shorter second remission and Continuous Treatment did not induce tumor resistance. Continuous Treatment may be essential, as patients with multiple myeloma will always have some residual disease. It should be noted that certain institutions including the Mayo Clinic cap Continuous/Maintenance treatment at approximately 2 years, due to the lack of randomized comparative data, on the value of prolonged maintenance beyond 2 years. Continuous Therapy Versus Fixed Duration of Therapy in Patients With Newly Diagnosed Multiple Myeloma. Palumbo A, Gay F, Cavallo F, et al. J Clin Oncol 2015;33:3459-3466
