SUMMARY: The American Cancer Society estimates that 49,670 people will be diagnosed with Oral cavity and Oropharyngeal cancer in 2017 and an estimated 9700 patients will die of the disease. Over 90% of these malignancies are Squamous Cell Carcinomas (SCCs). OroPharyngeal Squamous Cell Carcinoma (OPSCC) involves the tonsils and base of the tongue, and recent studies have shown that over 70% of these tumors are caused by Human Papilloma Virus (HPV) and HPV-16 is the predominant type present in the tumor cells. The CDC estimates that more than 2,370 new cases of Human Papilloma Virus associated OPSCC are diagnosed in women and nearly 9,356 are diagnosed in men, each year in the United States, and this incidence has been on the rise.
The American Society for Radiation Oncology (ASTRO) convened the OroPharyngeal Squamous Cell Carcinoma (OPSCC) Guideline Panel which consisted of a multidisciplinary team of radiation, medical, and surgical oncologists, to perform a systematic literature review of studies published from January 1990 through December 2014, in an attempt to present evidence-based guidelines, for the treatment of OPSCC, with definitive or adjuvant Radiation Therapy (RT).
The following are the key questions and recommendations of the Guideline panel.
(1) When is it appropriate to add systemic therapy to definitive RT in the treatment of OPSCC?
Stage IVA-B disease
A) Patients with stage IVA-B tumors receiving definitive RT should receive concurrent high-dose intermittent Cisplatin.
B) Patients who are medically unfit for high-dose Cisplatin should receive concurrent Cetuximab or Carboplatin-Fluorouracil.
C) Weekly Cisplatin may be considered for patients who are medically unfit for high-dose Cisplatin.
D) Concurrent Cetuximab should not be administered in combination with chemotherapy.
E) Intra-arterial chemotherapy should not be used in this patient group.
Stage III disease
A) Patients with T3 N0-1 tumors should receive concurrent systemic therapy.
B) Patients with T1-T2 N1 tumors who are at a significant risk for locoregional recurrence may be considered for concurrent systemic therapy.
Stage I to II disease
Concurrent systemic therapy is not recommended for this patient group as there is no evidence supporting the use of systemic therapy in this generally favorable population.
(2) When is it appropriate to deliver PostOperative RT (PORT) with and without systemic therapy following primary surgery for OPSCC?
A) Concurrent high-dose intermittent Cisplatin should be delivered with PORT to patients with positive surgical margins and/or extracapsular nodal extension, independent of HPV status or the extent of extranodal tumor.
B) Concurrent weekly Cisplatin may be administered with PORT to patients who are considered inappropriate for standard high-dose intermittent Cisplatin, after a careful discussion of patient preferences and the limited evidence supporting this treatment schedule.
C) For the high-risk postoperative patient unable to receive Cisplatin-based concurrent chemoradiation therapy, RT alone should be routinely delivered without concurrent systemic therapy. Given the limited evidence supporting alternative regimens, treatment with non-Cisplatin systemic therapy should be accompanied by a careful discussion of the risks and unknown benefits of the combination.
D) Patients treated with PORT should not receive concurrent weekly Carboplatin, weekly Docetaxel or Cetuximab, either alone or in combination with chemotherapy, although such regimens are currently under investigation.
E) Patients treated with PORT should not receive concurrent Mitomycin-C, alone or with Bleomycin, given the limited evidence and experience supporting its use.
F) Postoperative chemotherapy should not be delivered alone or sequentially with postoperative RT.
Intermediate-risk pathologic factors such as lymphovascular invasion (LVI), perineural invasion (PNI), T3-4 disease, or positive lymph nodes
A) These patients should not routinely receive concurrent systemic therapy with PORT.
B) Concurrent Cisplatin-based chemotherapy may be considered if the post operative pathologic findings suggest significant risk of locoregional recurrence.
C) PORT should be delivered to patients with pathologic T3 or T4 and pathologic N2 or N3 disease.
D) PORT may be delivered to patients with pathologic N1 disease after a careful discussion with the patient.
E) PORT may be delivered to patients with LVI and/or PNI as the only risk factor(s), after a careful discussion with the patient.
No pathologic risk factors
PORT may be delivered to patients without conventional adverse pathologic risk factors only if the clinical and surgical findings imply a particularly significant risk of locoregional recurrence and after a careful discussion with the patient.
(3) When is it appropriate to use induction chemotherapy in the treatment of OPSCC?
Induction Chemotherapy should not be routinely delivered to patients with OPSCC.
(4) What are the appropriate dose, fractionation, and volume regimens with and without systemic therapy in the treatment of OPSCC?
A) For patients with stage III-IV disease a dose of 70 Gy over 7 weeks should be delivered to gross primary and nodal disease.
B) The biologically equivalent dose of approximately 50 Gy in 2 Gy fractions or slightly higher should be delivered electively to clinically and radiographically negative regions at-risk for microscopic spread of tumor.
C) Altered fractionation should be used in patients with stage IVA-B disease treated with definitive RT, who are not receiving concurrent systemic therapy as well as patients with T3 N0–1 disease not receiving concurrent chemoradiation. Additionally, it may be considered for patients with T1–2 N1 or T2 N0 disease at high risk for recurrence.
D) When treating OPSCC with concurrent systemic therapy,, either standard or accelerated fractionation may be implemented.
Adjuvant PORT
Adjuvant PORT should be delivered to regions of microscopically positive primary site surgical margins and extracapsular nodal extension at 2 Gy/fraction once daily to a total dose between 60 and 66 Gy.
Early T-stage tonsillar carcinoma
A) Unilateral RT should be delivered to patients with well-lateralized (confined to tonsillar fossa) T1-T2 tonsillar cancer and N0-N1 nodal category.
B) Unilateral RT may be delivered to patients with lateralized (<1 cm of soft palate extension but without base of tongue involvement) T1-T2 N0-N2a tonsillar cancer, without clinical or radiographic evidence of extracapsular extension, after careful discussion of patient preferences and the relative benefits of unilateral treatment versus the potential for contralateral nodal recurrence and subsequent salvage treatment.
Radiation therapy for oropharyngeal squamous cell carcinoma: Executive summary of an ASTRO Evidence-Based Clinical Practice Guideline. Sher DJ, Adelstein DJ, Bajaj GK, et al. DOI: http://dx.doi.org/10.1016/j.prro.2017.02.002

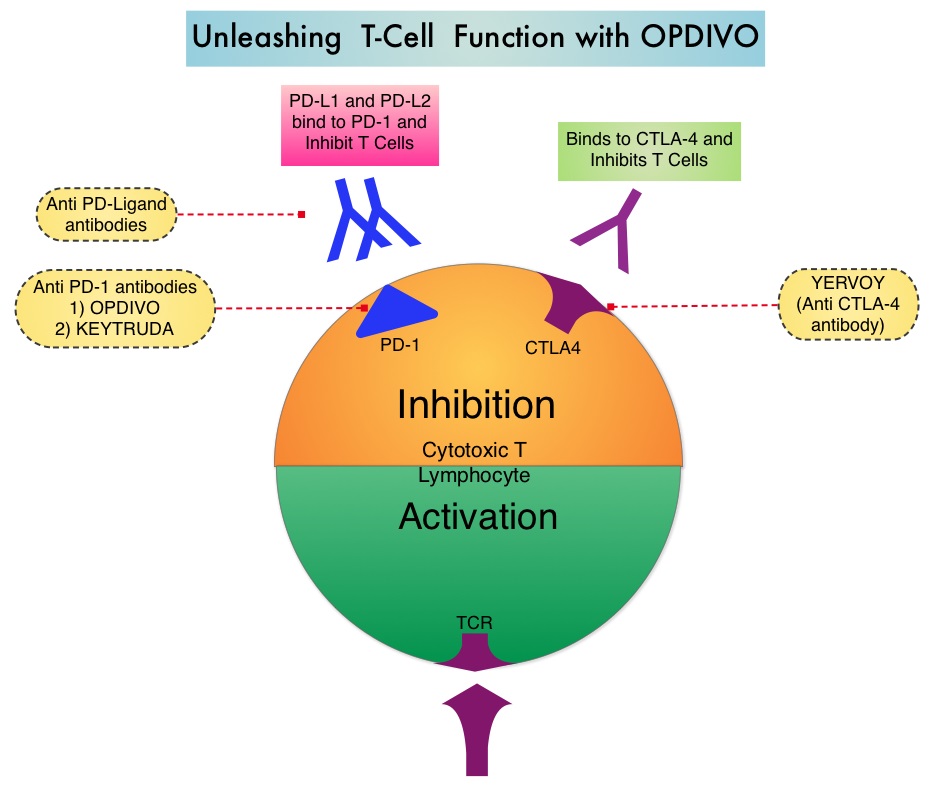
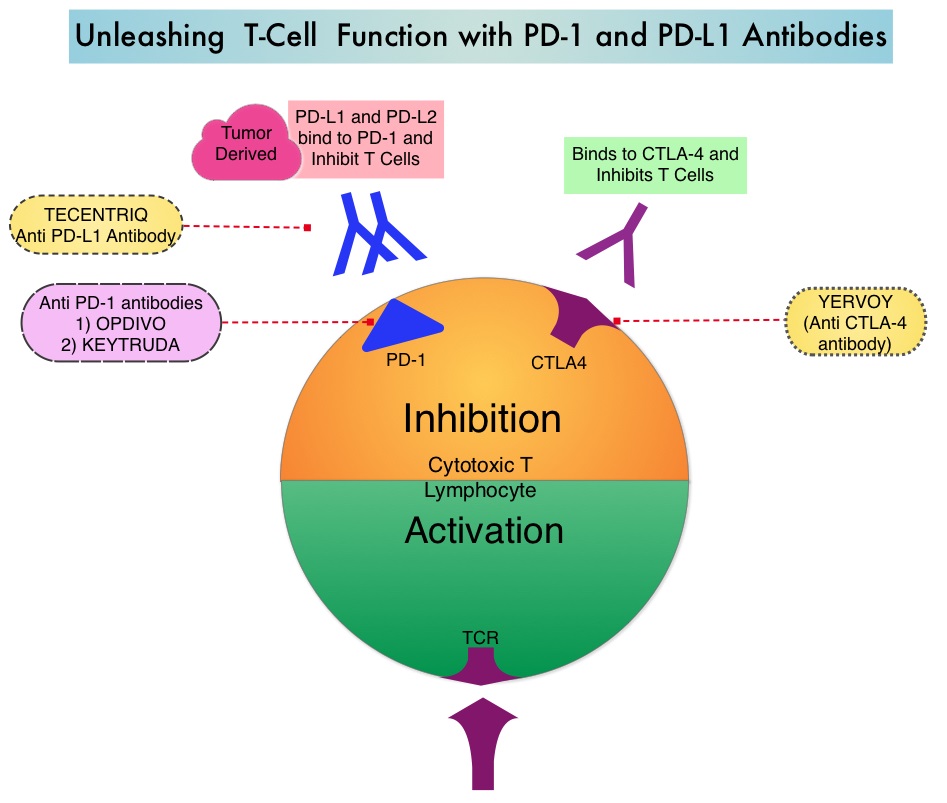 The treatment paradigm for solid tumors has been rapidly evolving with a better understanding of the Immune checkpoints or gate keepers. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent related to their ability to escape immune surveillance by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response.
The treatment paradigm for solid tumors has been rapidly evolving with a better understanding of the Immune checkpoints or gate keepers. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent related to their ability to escape immune surveillance by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response.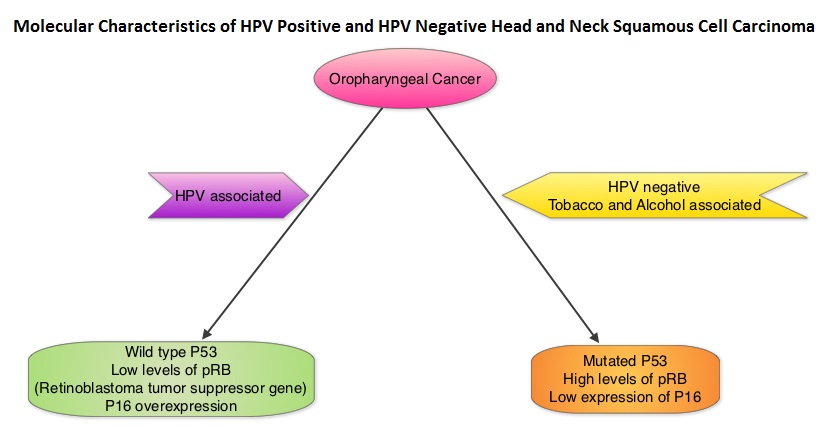
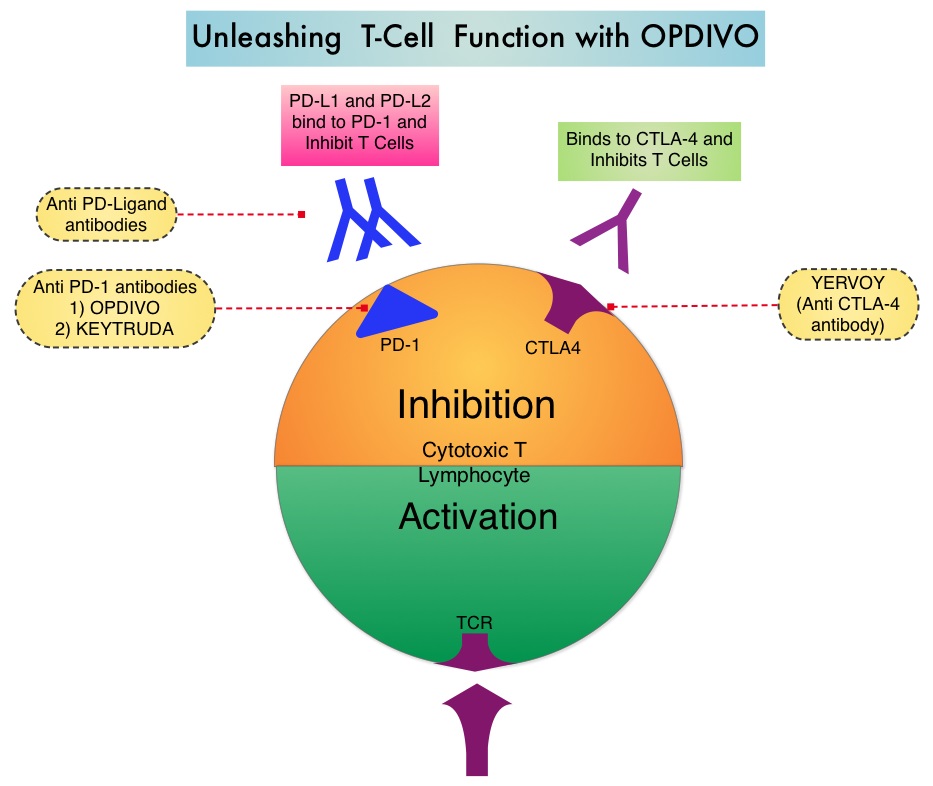
 Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent, related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4), also known as CD152, PD-1(Programmed cell Death-1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. The first Immune checkpoint protein to be clinically targeted was CTLA-4. YERVOY® (Ipilimumab), an antibody that blocks Immune checkpoint protein/receptor CTLA- 4, has been shown to prolong overall survival in patients with previously treated, unresectable or metastatic melanoma. KEYTRUDA® (Pembrolizumab) is a fully humanized, Immunoglobulin G4, monoclonal antibody, that binds to the PD-1 receptor and blocks its interaction with ligands PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the tumor-specific effector T cells. The FDA granted accelerated approval to KEYTRUDA® in September 2014, for the treatment of patients with unresectable or metastatic melanoma and disease progression following YERVOY® and, if BRAF V600 mutation positive, a BRAF inhibitor. The activity of KEYTRUDA® as a single agent in advanced SCCHN patients, was previously published and was noted in PD-L1 positive tumors, regardless of the Human PapillomaVirus (HPV) status. The Overall Response Rate in this patient group was 20% and 29% of patients had stable disease. The authors in this study reported the efficacy of once every three week dose of KEYTRUDA®, in a larger expansion cohort of KEYNOTE 012 study. In this study, 132 patients with recurrent/metastatic SCCHN were enrolled, regardless of their PD-L1 expression or HPV status. These patients received a fixed dose of KEYTRUDA® 200 mg IV, every 3 weeks and patients were evaluated every 8 weeks with radiographic imaging. The mean age was 59 years and 57% of the patients had 2 or more lines of therapy for recurrent disease. Treatment was continued until disease progression. The primary end point was Overall Response Rate (ORR) and secondary endpoints included Progression Free Survival (PFS) and Overall Survival (OS). The Overall Response Rate was 25% and stable disease was noted in an additional 25% of the patients. This amounted to a disease control rate of 50%. Patients with HPV-positive disease had a response rate of 20.6% and patients with HPV-negative disease had a response rate of 27.2%, suggesting that KEYTRUDA® was active in both subgroups of patients. Serious toxicities were reported in fewer than 10% of patients and the most common adverse event was fatigue (15.2%). The authors concluded that KEYTRUDA® given every 3 weeks was well tolerated and demonstrated a meaningful response rate in a heavily pretreated population of patients, with recurrent/metastatic SCCHN. Evaluation of PD-L1 status for this patient group is ongoing. Antitumor activity and safety of pembrolizumab in patients (pts) with advanced squamous cell carcinoma of the head and neck (SCCHN): Preliminary results from KEYNOTE-012 expansion cohort. Seiwert TY, Haddad RI, Gupta S, et al. J Clin Oncol 33, 2015 (suppl; abstr LBA6008)</s
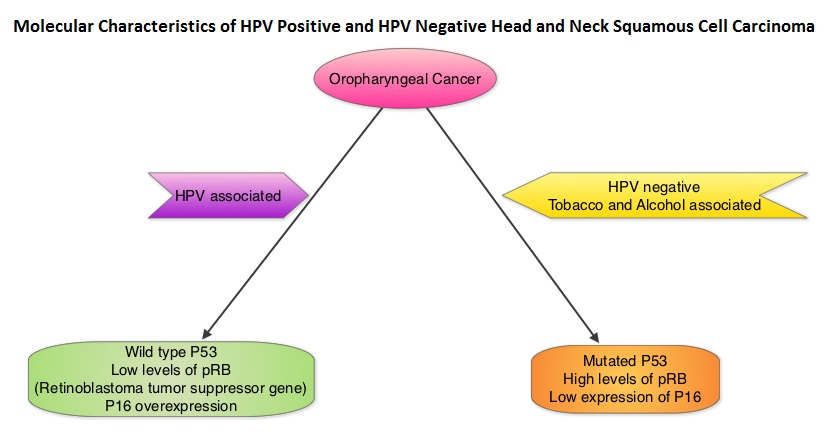
Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent, related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4), also known as CD152, PD-1(Programmed cell Death-1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. The first Immune checkpoint protein to be clinically targeted was CTLA-4. YERVOY® (Ipilimumab), an antibody that blocks Immune checkpoint protein/receptor CTLA- 4, has been shown to prolong overall survival in patients with previously treated, unresectable or metastatic melanoma. KEYTRUDA® (Pembrolizumab) is a fully humanized, Immunoglobulin G4, monoclonal antibody, that binds to the PD-1 receptor and blocks its interaction with ligands PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the tumor-specific effector T cells. The FDA granted accelerated approval to KEYTRUDA® in September 2014, for the treatment of patients with unresectable or metastatic melanoma and disease progression following YERVOY® and, if BRAF V600 mutation positive, a BRAF inhibitor. The activity of KEYTRUDA® as a single agent in advanced SCCHN patients, was previously published and was noted in PD-L1 positive tumors, regardless of the Human PapillomaVirus (HPV) status. The Overall Response Rate in this patient group was 20% and 29% of patients had stable disease. The authors in this study reported the efficacy of once every three week dose of KEYTRUDA®, in a larger expansion cohort of KEYNOTE 012 study. In this study, 132 patients with recurrent/metastatic SCCHN were enrolled, regardless of their PD-L1 expression or HPV status. These patients received a fixed dose of KEYTRUDA® 200 mg IV, every 3 weeks and patients were evaluated every 8 weeks with radiographic imaging. The mean age was 59 years and 57% of the patients had 2 or more lines of therapy for recurrent disease. Treatment was continued until disease progression. The primary end point was Overall Response Rate (ORR) and secondary endpoints included Progression Free Survival (PFS) and Overall Survival (OS). The Overall Response Rate was 25% and stable disease was noted in an additional 25% of the patients. This amounted to a disease control rate of 50%. Patients with HPV-positive disease had a response rate of 20.6% and patients with HPV-negative disease had a response rate of 27.2%, suggesting that KEYTRUDA® was active in both subgroups of patients. Serious toxicities were reported in fewer than 10% of patients and the most common adverse event was fatigue (15.2%). The authors concluded that KEYTRUDA® given every 3 weeks was well tolerated and demonstrated a meaningful response rate in a heavily pretreated population of patients, with recurrent/metastatic SCCHN. Evaluation of PD-L1 status for this patient group is ongoing. Antitumor activity and safety of pembrolizumab in patients (pts) with advanced squamous cell carcinoma of the head and neck (SCCHN): Preliminary results from KEYNOTE-012 expansion cohort. Seiwert TY, Haddad RI, Gupta S, et al. J Clin Oncol 33, 2015 (suppl; abstr LBA6008)</s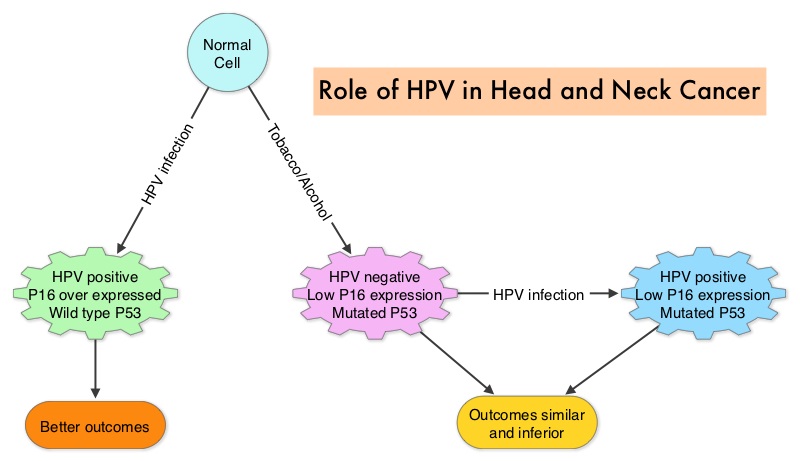 The malignant behavior of these tumors is dependent on the expression of viral E6 and E7 oncoproteins that inactivate the tumor suppressor proteins p53 and the retinoblastoma protein (pRB), respectively. HPV positive OroPharyngeal Squamous Cell Carcinoma (OPSCC) is more common among never smokers or light smokers and patients tend to be younger with better performance status. The pathobiology of HPV positive OroPharyngeal Squamous Cell Carcinoma (OPSCC) is different from the HPV negative OroPharyngeal Squamous Cell Carcinoma in that p53 is degraded/inactivated by viral E6 oncoprotein instead of by genetic mutation, pRB pathway is inactivated by viral E7 oncoprotein instead of Cyclin D1 amplification and p16 is overexpressed or upregulated instead of inactivation resulting from reduced negative feedback from pRB. Testing for HPV is based on several methodologies which include detection of HPV DNA by in situ hybridization (ISH) or Polymerase Chain Reaction (PCR), detection of HPV E6/E7 RNA expression by quantitative Reverse Transcriptase–PCR (qRT-PCR) and p16 protein overexpression, a surrogate marker of oncogenic HPV infection, by ImmunoHistoChemistry (IHC) staining. Both p16 overexpression testing by IHC and HPV by ISH can be easily performed on Formalin-Fixed Paraffin Embedded (FFPE) specimen whereas detection of HPV E6/E7 RNA expression, which is indicative of active viral oncogene transcription in tumor cells and is considered to be a gold standard, requires a larger tumor specimen compared with other methodologies.
The malignant behavior of these tumors is dependent on the expression of viral E6 and E7 oncoproteins that inactivate the tumor suppressor proteins p53 and the retinoblastoma protein (pRB), respectively. HPV positive OroPharyngeal Squamous Cell Carcinoma (OPSCC) is more common among never smokers or light smokers and patients tend to be younger with better performance status. The pathobiology of HPV positive OroPharyngeal Squamous Cell Carcinoma (OPSCC) is different from the HPV negative OroPharyngeal Squamous Cell Carcinoma in that p53 is degraded/inactivated by viral E6 oncoprotein instead of by genetic mutation, pRB pathway is inactivated by viral E7 oncoprotein instead of Cyclin D1 amplification and p16 is overexpressed or upregulated instead of inactivation resulting from reduced negative feedback from pRB. Testing for HPV is based on several methodologies which include detection of HPV DNA by in situ hybridization (ISH) or Polymerase Chain Reaction (PCR), detection of HPV E6/E7 RNA expression by quantitative Reverse Transcriptase–PCR (qRT-PCR) and p16 protein overexpression, a surrogate marker of oncogenic HPV infection, by ImmunoHistoChemistry (IHC) staining. Both p16 overexpression testing by IHC and HPV by ISH can be easily performed on Formalin-Fixed Paraffin Embedded (FFPE) specimen whereas detection of HPV E6/E7 RNA expression, which is indicative of active viral oncogene transcription in tumor cells and is considered to be a gold standard, requires a larger tumor specimen compared with other methodologies.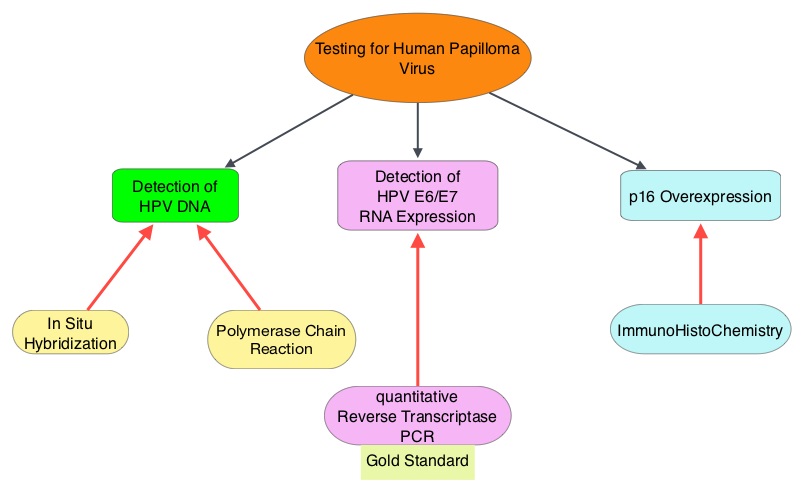 The concordance rate between HPV by ISH and p16 by IHC is approximately 90% in OPSCC, whereas the prevalence and significance p16 by IHC has remained unclear for HPV infection in non-OPSCC (oral cavity, hypopharynx and larynx). It is well established however that patients with HPV-positive/p16-positive OPSCC have better outcomes compared with those with HPV-negative/p16-negative OPSCC. However, the prognostic significance of p16 overexpression in non-OPSCC patients with or without evidence of HPV infection has not been clear. The authors in this study evaluated p16 protein overexpression by IHC and HPV status by HPV ISH as potential prognostic biomarkers in non-OPSCC tumors of patients enrolled in three prospective Radiation Therapy Oncology Group (RTOG) clinical trials. Of the 1921 patient enrolled in these three trials, 683 patients with non-OPSCC tumors were eligible and 52% (N=356) of these patients were tested for p16 overexpression and overall, 19.3% were p16 positive. When OPSCC and non-OPSCC patients were compared, OPSCC patients whose tumors were p16 positive had better Progression Free Survival and Overall Survival than non-OPSCC patients with p16 positive tumors. However, patients with p16 negative OPSCC and non-OPSCC have similar inferior outcomes. The authors concluded that patients with p16 negative non-OPSCC have worse outcomes than patients with p16 positive non-OPSCC, similar to that seen in patients with OPSCC and therefore HPV infection may influence outcomes in a subset of patients with non-OPSCC as well. Better methodologies for HPV detection and correlation with p16 expression will help identify “true” HPV infection related non-OPSCC and thereby enable tailored and less intense treatment, for this favorable group of patients. Chung CH, Zhang Q, Kong CS, et al. J Clin Oncol 2014; 32:3930-3938
The concordance rate between HPV by ISH and p16 by IHC is approximately 90% in OPSCC, whereas the prevalence and significance p16 by IHC has remained unclear for HPV infection in non-OPSCC (oral cavity, hypopharynx and larynx). It is well established however that patients with HPV-positive/p16-positive OPSCC have better outcomes compared with those with HPV-negative/p16-negative OPSCC. However, the prognostic significance of p16 overexpression in non-OPSCC patients with or without evidence of HPV infection has not been clear. The authors in this study evaluated p16 protein overexpression by IHC and HPV status by HPV ISH as potential prognostic biomarkers in non-OPSCC tumors of patients enrolled in three prospective Radiation Therapy Oncology Group (RTOG) clinical trials. Of the 1921 patient enrolled in these three trials, 683 patients with non-OPSCC tumors were eligible and 52% (N=356) of these patients were tested for p16 overexpression and overall, 19.3% were p16 positive. When OPSCC and non-OPSCC patients were compared, OPSCC patients whose tumors were p16 positive had better Progression Free Survival and Overall Survival than non-OPSCC patients with p16 positive tumors. However, patients with p16 negative OPSCC and non-OPSCC have similar inferior outcomes. The authors concluded that patients with p16 negative non-OPSCC have worse outcomes than patients with p16 positive non-OPSCC, similar to that seen in patients with OPSCC and therefore HPV infection may influence outcomes in a subset of patients with non-OPSCC as well. Better methodologies for HPV detection and correlation with p16 expression will help identify “true” HPV infection related non-OPSCC and thereby enable tailored and less intense treatment, for this favorable group of patients. Chung CH, Zhang Q, Kong CS, et al. J Clin Oncol 2014; 32:3930-3938
 To address this further, the authors examined the influence of tumor HPV status (tumor p16) on patterns of failure and survival after disease progression, by performing a retrospective analysis of the association between tumor p16 status and overall survival (OS) after disease progression, amongst patients enrolled in the Radiation Therapy Oncology Group (RTOG) trials 0129 and RTOG 0522. Eligible patients from these two trials had stage III-IV OroPharygeal Squamous Cell Carcinoma. Their tumor p16 status was known and these patients had local, regional, and/or distant progression after receiving platinum-based chemoradiotherapy. Of the 181 patients included in this analysis, 105 patients had p16 positive OPSCC and 76 patients had p16 negative OPSCC. Tumor p16 expression was evaluated using immunohistochemistry methodology. Even though the patterns of failure and median time to progression were similar for patients with p16-positive and p16-negative tumors, after a median follow up period of 4 years after disease progression, patients with p16-positive OPSCC had significantly improved Overall Survival (OS) rates compared with p16-negative patients (2-year OS, 54.6% vs 27.6%; median, 2.6 vs 0.8 years; P <0.001). This meant that patients with HPV positive recurrent OPSCC had nearly twice the overall survival rate compared to HPV negative patients. Further, salvage surgery after disease progression in HPV positive patients reduced the risk of death. Factors associated with improved survival in multivariate analysis included, HPV positive status, salvage surgery, locoregional progression vs distant progression, lower T stage at enrollment and less than 20 pack year smoking history.
To address this further, the authors examined the influence of tumor HPV status (tumor p16) on patterns of failure and survival after disease progression, by performing a retrospective analysis of the association between tumor p16 status and overall survival (OS) after disease progression, amongst patients enrolled in the Radiation Therapy Oncology Group (RTOG) trials 0129 and RTOG 0522. Eligible patients from these two trials had stage III-IV OroPharygeal Squamous Cell Carcinoma. Their tumor p16 status was known and these patients had local, regional, and/or distant progression after receiving platinum-based chemoradiotherapy. Of the 181 patients included in this analysis, 105 patients had p16 positive OPSCC and 76 patients had p16 negative OPSCC. Tumor p16 expression was evaluated using immunohistochemistry methodology. Even though the patterns of failure and median time to progression were similar for patients with p16-positive and p16-negative tumors, after a median follow up period of 4 years after disease progression, patients with p16-positive OPSCC had significantly improved Overall Survival (OS) rates compared with p16-negative patients (2-year OS, 54.6% vs 27.6%; median, 2.6 vs 0.8 years; P <0.001). This meant that patients with HPV positive recurrent OPSCC had nearly twice the overall survival rate compared to HPV negative patients. Further, salvage surgery after disease progression in HPV positive patients reduced the risk of death. Factors associated with improved survival in multivariate analysis included, HPV positive status, salvage surgery, locoregional progression vs distant progression, lower T stage at enrollment and less than 20 pack year smoking history.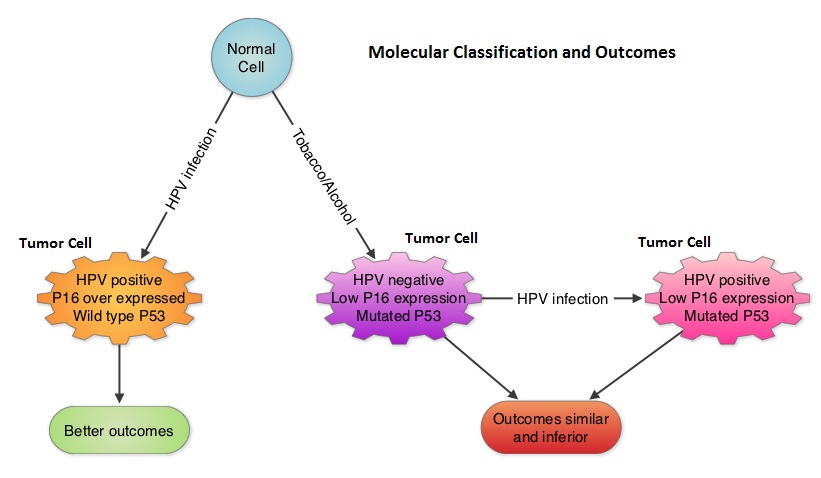 The authors concluded that patients with HPV positive OroPharyngeal Squamous Cell Carcinoma (OPSCC) have tumors with different molecular behavior, compared to HPV negative and tobacco related OPSCC and tumor p16 status is independently associated with Overall Survival among OPSCC patients with disease progression. This important information should be a stratification factor for clinical trials and the benefits of salvage surgery dictates close surveillance as these patients are followed up. Fakhry C, Zhang Q, Nguyen-Tan PF, et al. Published Ahead of Print on June 23, 2014 as 10.1200/JCO.2014.55.1937
The authors concluded that patients with HPV positive OroPharyngeal Squamous Cell Carcinoma (OPSCC) have tumors with different molecular behavior, compared to HPV negative and tobacco related OPSCC and tumor p16 status is independently associated with Overall Survival among OPSCC patients with disease progression. This important information should be a stratification factor for clinical trials and the benefits of salvage surgery dictates close surveillance as these patients are followed up. Fakhry C, Zhang Q, Nguyen-Tan PF, et al. Published Ahead of Print on June 23, 2014 as 10.1200/JCO.2014.55.1937