SUMMARY: The American Cancer Society estimates that approximately 133,000 new cases of ColoRectal Cancer (CRC) will be diagnosed in the United States in 2015 and close to 50,000 are expected to die of the disease. Approximately 15-25% of the patients with CRC present with metastatic disease at the time of diagnosis (synchronous metastases) and 50-60% of the patients with CRC will develop metastatic disease during the course of their illness. Patients with metastatic CRC, whose disease has progressed after treatment with standard therapies, have limited therapeutic options available, to treat their disease. Even though the Epidermal Growth Factor Receptor (EGFR) has been reported to be over expressed in 50-85% of the ColoRectal tumors, the intensity of ImmunoHistoChemical staining of EGFR in these tumors is not predictive of treatment response with EGFR directed antibody therapy such as ERBITUX® (Cetuximab) and VECTIBIX® (Panitumumab) and EGFR ImmunoHistoChemical staining should therefore not be a part of testing. The most common RAS oncogenes in human cancer are HRAS, KRAS, and NRAS. Mutations in HRAS are not common in colon cancer whereas KRAS and NRAS mutations are seen in colon cancer and tend to be mutually exclusive. It is also known that mutations in BRAF gene, which is downstream from RAS, may confer poor prognosis in colon cancer, regardless of therapy. It is estimated that approximately 40% of mCRC tumors harbor KRAS mutations and several studies had shown that metastatic ColoRectal Cancer (mCRC) tumors of patients harboring mutations in codon 12 or 13 of exon 2 of the KRAS gene, do not benefit from therapy with monoclonal antibodies directed against EGFR, when used as monotherapy or combined with chemotherapy. More recent studies have shown that KRAS mutations outside of exon 2 and mutations in NRAS are also predictive of lack of benefit with EGFR directed therapy.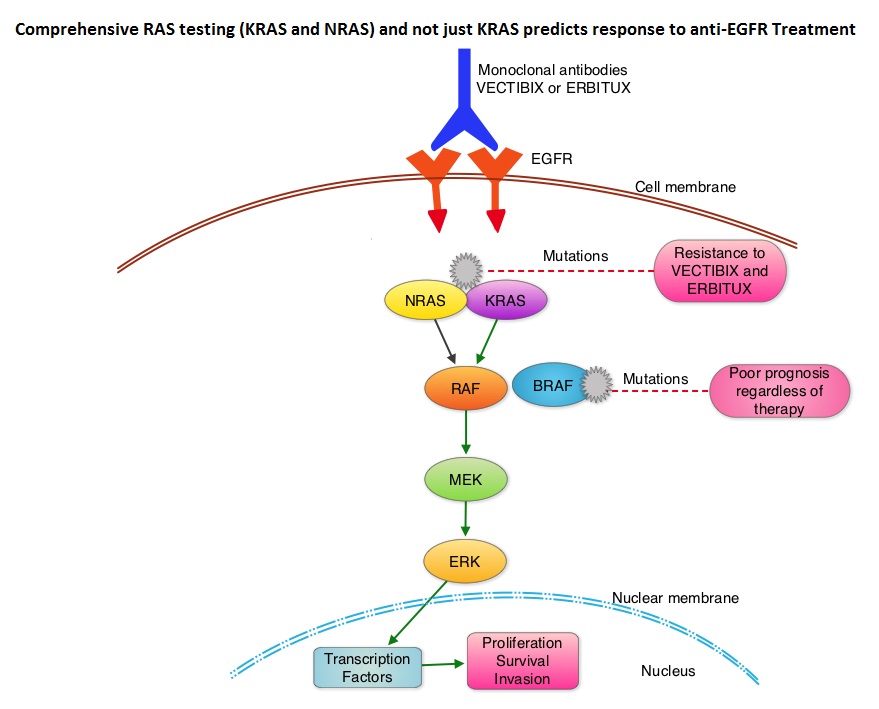
The American Society of Clinical Oncology Provisional Clinical Opinion (PCO) offers clinical direction for practicing oncology Health Care Providers, after publication or presentation of potentially practice-changing data from major studies. This 2015 ASCO Provisional Clinical Opinion update is a concerted effort of several organizations together with ASCO and they include the College of American Pathologists (CAP), the American Society for Clinical Pathology (ASCP), and the Association for Molecular Pathology (AMP). The Provisional Clinical Opinion (PCO) was released following inclusion of a systematic review of 11 meta-analyses, two retrospective analyses, and two health technology assessments in patients with mCRC. These studies evaluated the outcomes for patients with mCRC with no mutation detected or presence of mutation in additional exons in KRAS and NRAS. The extended RAS gene testing included
1) KRAS exons 2 (codons 12 and 13) exons 3 (codons 59 and 61) and exons 4 (codons 117 and 146)
2) NRAS exons 2 (codons 12 and 13), exons 3 (codons 59 and 61) and exons 4 (codons 117 and 146)
Two mCRC studies, the PRIME trial and the CRYSTAL trial, in their analysis included both KRAS and NRAS mutation data. In the PRIME study, the median Overall Survival (OS) in patients with wild-type RAS mCRC treated with VECTIBIX® plus FOLFOX was 26.0 months compared with 20.2 months with FOLFOX alone (HR=0.78; P=0.04). The Progression Free Survival (PFS) in patients not harboring RAS mutations was 10.1 months with VECTIBIX® plus FOLFOX, compared with 7.9 months with FOLFOX alone (HR=0.72; P=0.004). In the CRYSTAL trial, the median OS with ERBITUX® plus FOLFIRI was 28.4 months compared to 20.2 months with FOLFIRI alone, in patients with wild-type RAS mCRC (HR=0.69). The PFS in patients without RAS mutations was 11.4 months with ERBITUX® plus FOLFIRI compared with 8.4 months with FOLFIRI alone (HR, 0.56). Both these studies have shown that EGFR directed monoclonal antibody therapy does not benefit patients with KRAS or NRAS mutations and may even have a detrimental effect in these patients.
It was concluded that the current evidence indicates that EGFR directed therapy with monoclonal antibodies, ERBITUX® and VECTIBIX® should only be considered for treatment of patients with mCRC, whose tumors have no mutations detected after extended RAS mutation analysis. It is recommended that KRAS and NRAS genotyping of tumor should be performed at diagnosis of stage IV disease, as anti-EGFR directed therapy has no role in stage I, II or III disease. BRAF V600E mutation has been associated with poor prognosis in mCRC patients and may also predict lack of response to anti-EGFR monclonal antibody therapy, and should be a part of genotyping, at diagnosis of stage IV disease. Extended RAS Gene Mutation Testing in Metastatic Colorectal Carcinoma to Predict Response to Anti–Epidermal Growth Factor Receptor Monoclonal Antibody Therapy: American Society of Clinical Oncology Provisional Clinical Opinion Update 2015. Allegra CJ, Rumble RB, Hamilton SR, et al. Published online October 5, 2015. J Clin Oncol. doi: 10.1200/JCO.2015.63.9674.


 Survival of cancer cells in the human body may be to a significant extent, related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4), also known as CD152, PD-1(Programmed cell Death-1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic respons
Survival of cancer cells in the human body may be to a significant extent, related to their ability to escape immune surveillance, by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4), also known as CD152, PD-1(Programmed cell Death-1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic respons SIRFLOX is an International, multi-center, open-label, randomized phase III study, which evaluated the efficacy and safety of combining modified FOLFOX6 (Oxaliplatin, 5-FU and Leucovorin) chemotherapy regimen with or without AVASTIN® (Bevacizumab) with SIRT, using Y-90 resin microspheres, as first line treatment in patients with unresectable liver only or liver dominant metastatic ColoRectal Cancer (mCRC). The randomization included 530 patients of whom 263 patients received mFOLFOX6 with or without AVASTIN® (Group A) and 267 patients received mFOLFOX6 + SIRT administered once with cycle 1, with or without AVASTIN® (Group B), with the treatment given until disease progression. Patients were stratified based on the extent of liver involvement (25% or less versus more than 25%), presence of extra hepatic disease (liver only versus liver dominant disease) and treatment with AVASTIN®, which was at the discretion of the attending physician. Forty percent of the patients had extra hepatic disease. The primary endpoint was Progression Free Survival (PFS). With a median follow up of 36.1 months, the median PFS in the liver was 12.6 months versus 20.5 months in Group A versus Group B respectively (HR=0.69; P=0.002). The hepatic Response Rate was 68.8% versus 78.7% (P=0.042), with a Complete Response Rate of 1.9% versus 6.0% (P=0.02) in Groups A and B respectively. Even though hematologic and gastrointestinal adverse events were higher in the SIRT group, the toxicity levels were acceptable. The authors concluded that the addition of SIRT to chemotherapy resulted in a 7.9 month improvement in Progression Free Survival in the liver, for patients with unresectable metastatic ColoRectal cancer (mCRC), with a 31% reduction in the risk of tumor progression in the liver. With the liver being the most common site of spread in patients with metastatic CRC, this study provides Level One evidence to support the use of SIRT in combination with chemotherapy in this patient group. SIRFLOX: Randomized phase III trial comparing first-line mFOLFOX6 ± bevacizumab (bev) versus mFOLFOX6 + selective internal radiation therapy (SIRT) ± bev in patients (pts) with metastatic colorectal cancer (mCRC). Gibbs P, Heinemann V, Sharma NK, et al. J Clin Oncol 33, 2015 (suppl; abstr 3502)</s
SIRFLOX is an International, multi-center, open-label, randomized phase III study, which evaluated the efficacy and safety of combining modified FOLFOX6 (Oxaliplatin, 5-FU and Leucovorin) chemotherapy regimen with or without AVASTIN® (Bevacizumab) with SIRT, using Y-90 resin microspheres, as first line treatment in patients with unresectable liver only or liver dominant metastatic ColoRectal Cancer (mCRC). The randomization included 530 patients of whom 263 patients received mFOLFOX6 with or without AVASTIN® (Group A) and 267 patients received mFOLFOX6 + SIRT administered once with cycle 1, with or without AVASTIN® (Group B), with the treatment given until disease progression. Patients were stratified based on the extent of liver involvement (25% or less versus more than 25%), presence of extra hepatic disease (liver only versus liver dominant disease) and treatment with AVASTIN®, which was at the discretion of the attending physician. Forty percent of the patients had extra hepatic disease. The primary endpoint was Progression Free Survival (PFS). With a median follow up of 36.1 months, the median PFS in the liver was 12.6 months versus 20.5 months in Group A versus Group B respectively (HR=0.69; P=0.002). The hepatic Response Rate was 68.8% versus 78.7% (P=0.042), with a Complete Response Rate of 1.9% versus 6.0% (P=0.02) in Groups A and B respectively. Even though hematologic and gastrointestinal adverse events were higher in the SIRT group, the toxicity levels were acceptable. The authors concluded that the addition of SIRT to chemotherapy resulted in a 7.9 month improvement in Progression Free Survival in the liver, for patients with unresectable metastatic ColoRectal cancer (mCRC), with a 31% reduction in the risk of tumor progression in the liver. With the liver being the most common site of spread in patients with metastatic CRC, this study provides Level One evidence to support the use of SIRT in combination with chemotherapy in this patient group. SIRFLOX: Randomized phase III trial comparing first-line mFOLFOX6 ± bevacizumab (bev) versus mFOLFOX6 + selective internal radiation therapy (SIRT) ± bev in patients (pts) with metastatic colorectal cancer (mCRC). Gibbs P, Heinemann V, Sharma NK, et al. J Clin Oncol 33, 2015 (suppl; abstr 3502)</s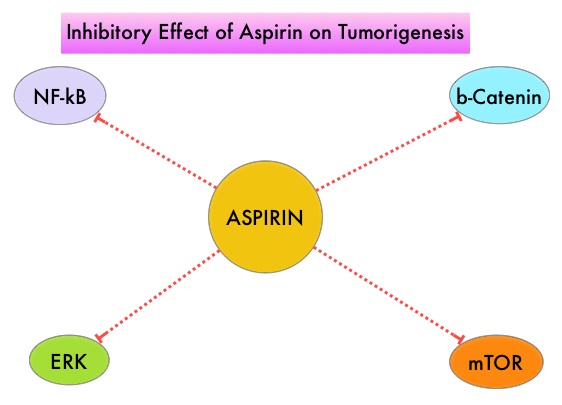 It is postulated that Aspirin also works by COX-independent mechanisms such as, the inhibition of NF-kB and Wnt/ β-catenin signaling, which may play a role in its chemopreventive properties. Even though the benefits of Aspirin in the primary prevention of CRC remains well established, the role of Aspirin in secondary prevention in patients with CRC is unclear. The authors conducted this trial to evaluate the association between Aspirin use after diagnosis of CRC with CRC-Specific Survival (CSS) and Overall Survival (OS) in the largest group of patients ever studied. The study authors in this retrospective study identified 25,644 patients in the Cancer Registry of Norway, diagnosed with ColoRectal Cancer (CRC) from 2004 through 2011. Using the Norwegian Prescription Database, the authors were then able to establish that 6,109 patients in this large cohort had documented exposure to Aspirin. Exposure to Aspirin was defined as a prescription for more than 6 months of Aspirin following a diagnosis of CRC. The median follow up was 2.2 years. The authors performed a multivariate regression analysis controlling for age, gender, tumor stage, tumor differentiation and noted that exposure to Aspirin post-diagnosis, independently improved ColoRectal Cancer (CRC) -Specific Survival (HR=0.75; P<0.001) and Overall Survival (HR=0.86; P<0.001). The authors concluded that in this large group of unselected ColoRectal Cancer (CRC) patients, exposure to Aspirin after the diagnosis of CRC is independently associated with improved Colorectal Cancer-Specific Survival and Overall Survival. They added that because of the risk of bleeding, the risk–benefit should be assessed before Aspirin is routinely recommended to this patient population. Impact of aspirin as secondary prevention in an unselected cohort of 25,644 patients with colorectal cancer: A population-based study. Bains S, Mahic M, Cvancarova M, et al. J Clin Oncol 33, 2015 (suppl; abstr 3504)
It is postulated that Aspirin also works by COX-independent mechanisms such as, the inhibition of NF-kB and Wnt/ β-catenin signaling, which may play a role in its chemopreventive properties. Even though the benefits of Aspirin in the primary prevention of CRC remains well established, the role of Aspirin in secondary prevention in patients with CRC is unclear. The authors conducted this trial to evaluate the association between Aspirin use after diagnosis of CRC with CRC-Specific Survival (CSS) and Overall Survival (OS) in the largest group of patients ever studied. The study authors in this retrospective study identified 25,644 patients in the Cancer Registry of Norway, diagnosed with ColoRectal Cancer (CRC) from 2004 through 2011. Using the Norwegian Prescription Database, the authors were then able to establish that 6,109 patients in this large cohort had documented exposure to Aspirin. Exposure to Aspirin was defined as a prescription for more than 6 months of Aspirin following a diagnosis of CRC. The median follow up was 2.2 years. The authors performed a multivariate regression analysis controlling for age, gender, tumor stage, tumor differentiation and noted that exposure to Aspirin post-diagnosis, independently improved ColoRectal Cancer (CRC) -Specific Survival (HR=0.75; P<0.001) and Overall Survival (HR=0.86; P<0.001). The authors concluded that in this large group of unselected ColoRectal Cancer (CRC) patients, exposure to Aspirin after the diagnosis of CRC is independently associated with improved Colorectal Cancer-Specific Survival and Overall Survival. They added that because of the risk of bleeding, the risk–benefit should be assessed before Aspirin is routinely recommended to this patient population. Impact of aspirin as secondary prevention in an unselected cohort of 25,644 patients with colorectal cancer: A population-based study. Bains S, Mahic M, Cvancarova M, et al. J Clin Oncol 33, 2015 (suppl; abstr 3504)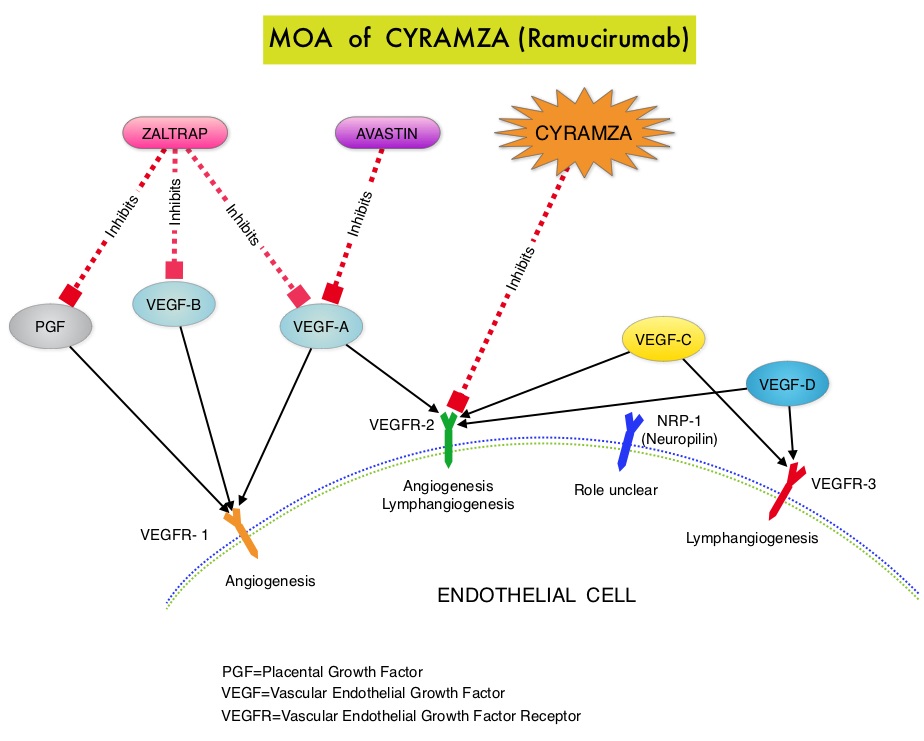 CYRAMZA® (Ramucirumab) is a recombinant human IgG1 monoclonal antibody that binds to Vascular Endothelial Growth Factor Receptor 2 (VEGFR-2) and blocks binding of the VEGFR ligands VEGF-A, VEGF-C, and VEGF-D and thus inhibits ligand-induced proliferation and migration of endothelial cells. This is unlike AVASTIN®, which inhibits VEGF-A. Several studies have demonstrated that continuing VEGF inhibition along with standard second-line chemotherapy beyond disease progression has a significant advantage, in patients with mCRC. Two observational studies (BRiTE and ARIES) as well as an open label phase III study have all shown improvement in median Overall Survival (OS) when AVASTIN® was continued beyond first progression and given along with second line chemotherapy. In the VELOUR trial, FOLFIRI in combination with ZALTRAP® (Aflibercept), a VEGF-A, VEGF-B and Placental Growth Factor inhibitor, when given as second line therapy for those patients with mCRC who had progressed on AVASTIN® plus ELOXATIN® containing regimen, resulted in a significant improvement in the median PFS and OS.
CYRAMZA® (Ramucirumab) is a recombinant human IgG1 monoclonal antibody that binds to Vascular Endothelial Growth Factor Receptor 2 (VEGFR-2) and blocks binding of the VEGFR ligands VEGF-A, VEGF-C, and VEGF-D and thus inhibits ligand-induced proliferation and migration of endothelial cells. This is unlike AVASTIN®, which inhibits VEGF-A. Several studies have demonstrated that continuing VEGF inhibition along with standard second-line chemotherapy beyond disease progression has a significant advantage, in patients with mCRC. Two observational studies (BRiTE and ARIES) as well as an open label phase III study have all shown improvement in median Overall Survival (OS) when AVASTIN® was continued beyond first progression and given along with second line chemotherapy. In the VELOUR trial, FOLFIRI in combination with ZALTRAP® (Aflibercept), a VEGF-A, VEGF-B and Placental Growth Factor inhibitor, when given as second line therapy for those patients with mCRC who had progressed on AVASTIN® plus ELOXATIN® containing regimen, resulted in a significant improvement in the median PFS and OS.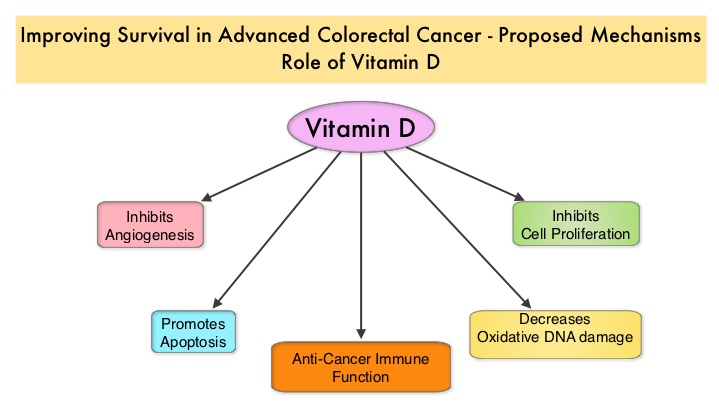 There appears to be a strong association between plasma 25(OH)D level and colorectal cancer (CRC) specific mortality, with better outcomes in patients with Stage I-III CRC, who had higher plasma levels of 25(OH)D (Zgaga L, et al. J Clin Oncol 2014;32:2430-2439). The researchers in this present study conducted a prospective analysis of data from CALGB 80405 trial and evaluated the relationship between plasma 25(OH)D level and patient outcomes, which included Overall Survival and Progression Free Survival (PFS). CALGB 80405 is a phase III trial in which patients with newly diagnosed, advanced colorectal cancer were initially randomized to three groups- 1) Chemotherapy (FOLFIRI or mFOLFOX6) with ERBITUX® (Cetuximab) 2) Chemotherapy with AVASTIN® (Bevacizumab) 3) Chemotherapy with ERBITUX® and AVASTIN®. The protocol was later amended to only include patients with KRAS Wild Type tumors and the chemotherapy with ERBITUX® and AVASTIN® group was deleted. This trial was not designed to compare chemotherapy regimens. The Overall Survival (OS) in both the treatment groups were similar at 29+ months.
There appears to be a strong association between plasma 25(OH)D level and colorectal cancer (CRC) specific mortality, with better outcomes in patients with Stage I-III CRC, who had higher plasma levels of 25(OH)D (Zgaga L, et al. J Clin Oncol 2014;32:2430-2439). The researchers in this present study conducted a prospective analysis of data from CALGB 80405 trial and evaluated the relationship between plasma 25(OH)D level and patient outcomes, which included Overall Survival and Progression Free Survival (PFS). CALGB 80405 is a phase III trial in which patients with newly diagnosed, advanced colorectal cancer were initially randomized to three groups- 1) Chemotherapy (FOLFIRI or mFOLFOX6) with ERBITUX® (Cetuximab) 2) Chemotherapy with AVASTIN® (Bevacizumab) 3) Chemotherapy with ERBITUX® and AVASTIN®. The protocol was later amended to only include patients with KRAS Wild Type tumors and the chemotherapy with ERBITUX® and AVASTIN® group was deleted. This trial was not designed to compare chemotherapy regimens. The Overall Survival (OS) in both the treatment groups were similar at 29+ months.