SUMMARY: The American Cancer Society estimates that approximately 133,000 new cases of ColoRectal Cancer (CRC) will be diagnosed in the United States in 2015 and close to 50,000 are expected to die of the disease. Approximately 15-25% of the patients with CRC present with metastatic disease at the time of diagnosis (synchronous metastases) and 50-60% of the patients with CRC will develop metastatic disease during the course of their illness. Patients with metastatic CRC, whose disease has progressed after treatment with standard therapies, have limited therapeutic options available, to treat their disease. Even though the Epidermal Growth Factor Receptor (EGFR) has been reported to be over expressed in 50-85% of the ColoRectal tumors, the intensity of ImmunoHistoChemical staining of EGFR in these tumors is not predictive of treatment response with EGFR directed antibody therapy such as ERBITUX® (Cetuximab) and VECTIBIX® (Panitumumab) and EGFR ImmunoHistoChemical staining should therefore not be a part of testing. The most common RAS oncogenes in human cancer are HRAS, KRAS, and NRAS. Mutations in HRAS are not common in colon cancer whereas KRAS and NRAS mutations are seen in colon cancer and tend to be mutually exclusive. It is also known that mutations in BRAF gene, which is downstream from RAS, may confer poor prognosis in colon cancer, regardless of therapy. It is estimated that approximately 40% of mCRC tumors harbor KRAS mutations and several studies had shown that metastatic ColoRectal Cancer (mCRC) tumors of patients harboring mutations in codon 12 or 13 of exon 2 of the KRAS gene, do not benefit from therapy with monoclonal antibodies directed against EGFR, when used as monotherapy or combined with chemotherapy. More recent studies have shown that KRAS mutations outside of exon 2 and mutations in NRAS are also predictive of lack of benefit with EGFR directed therapy.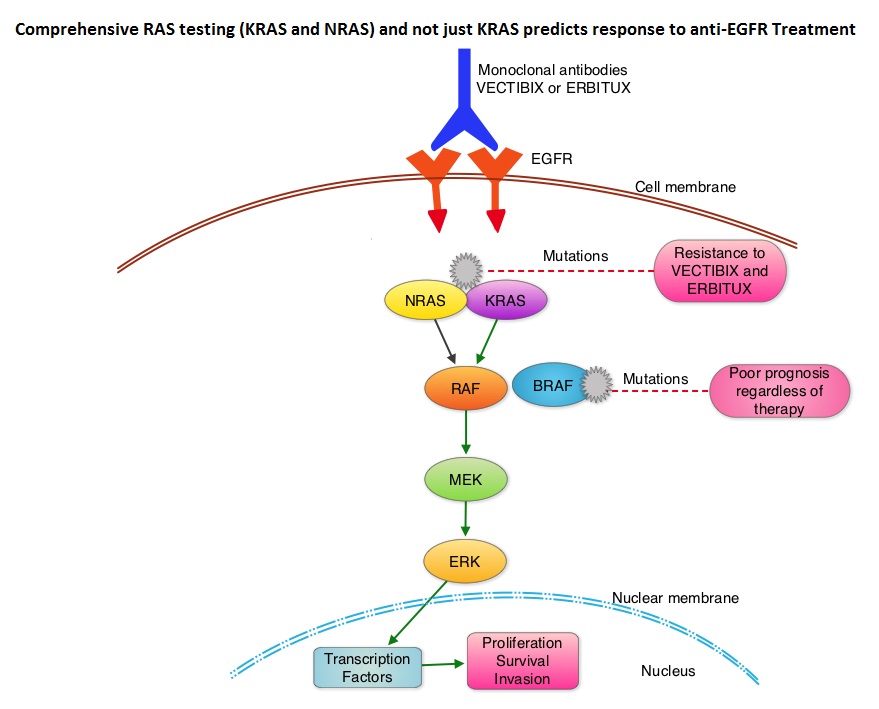
The American Society of Clinical Oncology Provisional Clinical Opinion (PCO) offers clinical direction for practicing oncology Health Care Providers, after publication or presentation of potentially practice-changing data from major studies. This 2015 ASCO Provisional Clinical Opinion update is a concerted effort of several organizations together with ASCO and they include the College of American Pathologists (CAP), the American Society for Clinical Pathology (ASCP), and the Association for Molecular Pathology (AMP). The Provisional Clinical Opinion (PCO) was released following inclusion of a systematic review of 11 meta-analyses, two retrospective analyses, and two health technology assessments in patients with mCRC. These studies evaluated the outcomes for patients with mCRC with no mutation detected or presence of mutation in additional exons in KRAS and NRAS. The extended RAS gene testing included
1) KRAS exons 2 (codons 12 and 13) exons 3 (codons 59 and 61) and exons 4 (codons 117 and 146)
2) NRAS exons 2 (codons 12 and 13), exons 3 (codons 59 and 61) and exons 4 (codons 117 and 146)
Two mCRC studies, the PRIME trial and the CRYSTAL trial, in their analysis included both KRAS and NRAS mutation data. In the PRIME study, the median Overall Survival (OS) in patients with wild-type RAS mCRC treated with VECTIBIX® plus FOLFOX was 26.0 months compared with 20.2 months with FOLFOX alone (HR=0.78; P=0.04). The Progression Free Survival (PFS) in patients not harboring RAS mutations was 10.1 months with VECTIBIX® plus FOLFOX, compared with 7.9 months with FOLFOX alone (HR=0.72; P=0.004). In the CRYSTAL trial, the median OS with ERBITUX® plus FOLFIRI was 28.4 months compared to 20.2 months with FOLFIRI alone, in patients with wild-type RAS mCRC (HR=0.69). The PFS in patients without RAS mutations was 11.4 months with ERBITUX® plus FOLFIRI compared with 8.4 months with FOLFIRI alone (HR, 0.56). Both these studies have shown that EGFR directed monoclonal antibody therapy does not benefit patients with KRAS or NRAS mutations and may even have a detrimental effect in these patients.
It was concluded that the current evidence indicates that EGFR directed therapy with monoclonal antibodies, ERBITUX® and VECTIBIX® should only be considered for treatment of patients with mCRC, whose tumors have no mutations detected after extended RAS mutation analysis. It is recommended that KRAS and NRAS genotyping of tumor should be performed at diagnosis of stage IV disease, as anti-EGFR directed therapy has no role in stage I, II or III disease. BRAF V600E mutation has been associated with poor prognosis in mCRC patients and may also predict lack of response to anti-EGFR monclonal antibody therapy, and should be a part of genotyping, at diagnosis of stage IV disease. Extended RAS Gene Mutation Testing in Metastatic Colorectal Carcinoma to Predict Response to Anti–Epidermal Growth Factor Receptor Monoclonal Antibody Therapy: American Society of Clinical Oncology Provisional Clinical Opinion Update 2015. Allegra CJ, Rumble RB, Hamilton SR, et al. Published online October 5, 2015. J Clin Oncol. doi: 10.1200/JCO.2015.63.9674.

