SUMMARY: ColoRectal Cancer (CRC) is the third most common cancer diagnosed in both men and women in the United States. The American Cancer Society estimates that approximately 135,430 new cases of ColoRectal Cancer will be diagnosed in the United States in 2017 and over 50,260 patients are expected to die of the disease. Patients with ColoRectal Carcinoma are often followed up with regular CEA measurements after curative surgical resection and a rising CEA may be the first sign of CRC recurrence and warrants further investigation. Approximately 50% of the patients with early-stage disease after surgical resection will relapse with metastatic disease, during the first 3 years of follow-up. They may present with synchronous disease defined as distant metastases occurring within 6 months, and metachronous disease defined as distant metastases occuring beyond 6 months, of the primary diagnosis of CRC. These patients with oligo-metastatic disease, when detected early, may be potentially curable.
The authors in this study sought to (a) evaluate the utility of PET-CT in detecting occult disease recurrence in patients with raised CEA and (b) establish the prognostic effects of early detection of disease recurrence in patients with CRC. This retrospective analysis screened1200 patients from 2004 to 2010, with a confirmed diagnosis of CRC, who on follow up after curative therapy underwent FDG PET-CT imaging, for an elevated CEA, after normal findings on conventional investigations. Patients who had already received treatment with curative intent for synchronous or metachronous oligo-metastatic disease, including surgical resection, radiofrequency ablation (RFA), and radical chemoradiation, were also included. An elevated CEA level was defined as more than 3 ng/mL in nonsmokers and more than 5 ng/mL in smokers. A minimum of clinical and radiological follow up for 12 months or histopathological confirmation, were required, to ascertain recurrent disease. Eighty eight (N=88) patients who underwent PET-CT imaging because of any clinical indication and met the eligibility criteria, were included in the study. The mean age of patients was 66 years and 59% were male.
Recurrent disease was confirmed in 64% of the patients within the 12 months after their FDG PET-CT scan and the PET scan was able to detect the site of subtle relapse. The sensitivity of PET-CT to detect recurrence was 88% and the specificity was 88% as well. Fifty five percent (55%) of the patients with PET-CT-detected relapsed disease were deemed eligible for further curative therapy of whom 70% went on to receive potentially curative therapy. The Positive Predictive Value and Negative Predictive Value for FDG PET-CT to predict recurrence were 93% and 80% respectively.
The median Time To Progression for patients who received potentially curative therapy for the PET-CT-detected recurrence was 8.8 months versus 2.2 months for the patients not treated with curative intent. The median Overall Survival was 39.9 months for those who received potentially curative treatment versus 15.6 months for those who did not receive curative therapy. The 5-year survival rate in the curative group was 36.8% versus 6.1% in the non-curative group (P <0.001).
The authors concluded that early use of FDG PET-CT in patients with rising CEA levels is a highly sensitive and specific tool for the detection of occult ColoRectal Cancer recurrence, and in more than 50% of these patients, recurrent disease may still be amenable to curative therapy, and long-term survival can be achieved in a subgroup of this patient population. Survival Outcomes in Asymptomatic Patients With Normal Conventional Imaging but Raised Carcinoembryonic Antigen Levels in Colorectal Cancer Following Positron Emission Tomography-Computed Tomography Imaging. Khana K, Athaudaa A, Aitken K, et al. The Oncologist 2016;21:1502-1508

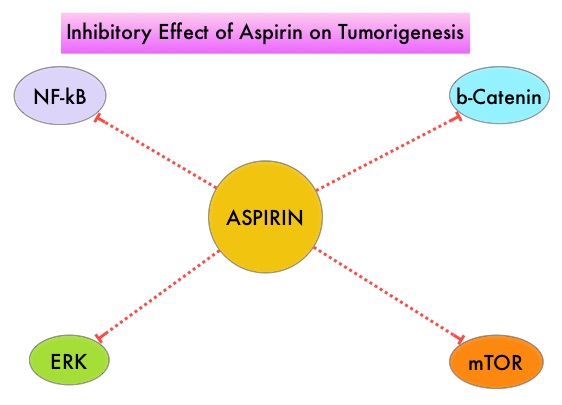
 Even though the benefits of Aspirin in the primary prevention of CRC remains well established, the role of Aspirin in secondary prevention in patients with CRC (after the diagnosis of CRC) is unclear. Platelets have long been implicated in the mechanism of tumor metastases. More recent data suggests that platelets may play a role in tumorigenesis as well, through the release of angiogenic and growth factors due to overexpression of COX-2. Daily low dose Aspirin inhibits COX-1 and COX-2. It is postulated that Aspirin also works by COX-independent mechanisms such as, the inhibition of NF-kB and Wnt/ β-catenin signaling, which may play a role in its chemopreventive properties.
Even though the benefits of Aspirin in the primary prevention of CRC remains well established, the role of Aspirin in secondary prevention in patients with CRC (after the diagnosis of CRC) is unclear. Platelets have long been implicated in the mechanism of tumor metastases. More recent data suggests that platelets may play a role in tumorigenesis as well, through the release of angiogenic and growth factors due to overexpression of COX-2. Daily low dose Aspirin inhibits COX-1 and COX-2. It is postulated that Aspirin also works by COX-independent mechanisms such as, the inhibition of NF-kB and Wnt/ β-catenin signaling, which may play a role in its chemopreventive properties.