SUMMARY: ColoRectal Cancer (CRC) is the third most common cancer diagnosed in both men and women in the United States. The American Cancer Society estimates that approximately 140,250 new cases of CRC will be diagnosed in the United States in 2018 and about 50,630 patients are expected to die of the disease. The lifetime risk of developing CRC is about 1 in 21 (4.7%).
The DNA MisMatchRepair (MMR) system is responsible for molecular surveillance and works as an editing tool that identifies errors within the microsatellite regions of DNA and removes them. Defective MMR system leads to MSI (Micro Satellite Instability) and hypermutation, triggering an enhanced antitumor immune response. MSI (Micro Satellite Instability) is therefore a hallmark of defective/deficient DNA MisMatchRepair (dMMR) system and occurs in 15% of all colorectal cancers. Defective MisMatchRepair can be a sporadic or heritable event. Approximately 65% of the MSI tumors are sporadic and when sporadic, the DNA MisMatchRepair gene is MLH1. Defective MisMatchRepair can also manifest as a germline mutation occurring in 1 of the 4 MisMatchRepair genes which include MLH1, MSH2, MSH6, PMS2. This produces Lynch Syndrome (Hereditary Nonpolyposis Colorectal Carcinoma – HNPCC), an Autosomal Dominant disorder and is the most common form of hereditary colon cancer, accounting for 35% of the MSI colorectal cancers. MSI tumors tend to have better outcomes and this has been attributed to the abundance of tumor infiltrating lymphocytes in these tumors from increase immunogenicity. These tumors therefore are susceptible to PD-1 blockade with immune checkpoint inhibitors.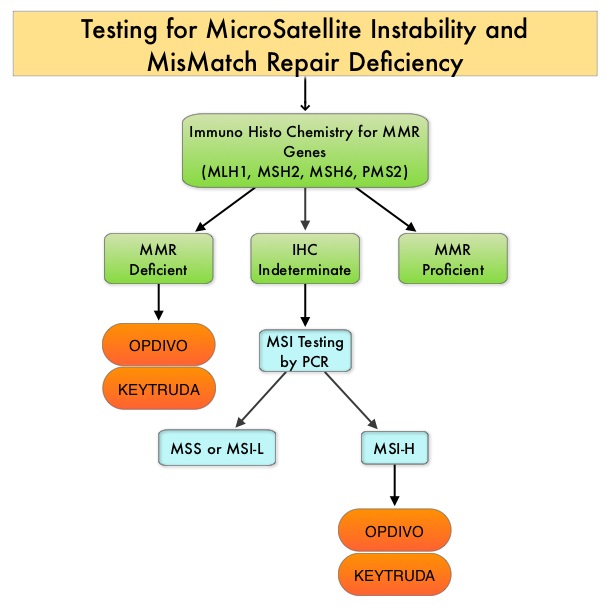
MSI (Micro Satellite Instability) testing is performed using a PCR based assay and MSI-High refers to instability at 2 or more of the 5 mononucleotide repeat markers and MSI-Low refers to instability at 1 of the 5 markers. Patients are considered Micro Satellite Stable (MSS) if no instability occurs. MSI-L and MSS are grouped together because MSI-L tumors are uncommon and behave similar to MSS tumors. Tumors considered MSI-H have deficiency of one or more of the DNA MisMatchRepair genes. MMR gene deficiency can be detected by ImmunoHistoChemistry (IHC). MLH1 gene is often lost in association with PMS2. NCCN Guidelines recommend MMR or MSI testing for all patients with a history of Colon or Rectal cancer.
CheckMate 142 is a large, multi-center, open label, phase II trial which evaluated the efficacy and safety of PD-1 inhibitor treatment in patients with dMMR/MSI-H metastatic colorectal cancer. This study evaluated the benefit of OPDIVO® alone or in combination YERVOY® in this patient population. The rationale behind combining OPDIVO® a PD-1 inhibitor and YERVOY®, a CTLA-4 inhibitor, was based on the synergy between these two agents, to promote T-cell antitumor activity, thereby improving upon single-agent activity of OPDIVO®. The study enrolled 119 patients who received OPDIVO® as a single agent at 3 mg/kg IV every 2 weeks or OPDIVO® 3 mg/kg plus YERVOY® 1 mg/kg every 3 weeks for 4 doses, followed by OPDIVO® 3 mg/kg every 2 weeks. Treatment was continued until disease progression or unacceptable toxicities. The Primary endpoint was Objective Response Rate (ORR) and exploratory endpoints included Safety, Progression Free Survival (PFS), Overall Survival (OS) and efficacy in biomarker-defined populations. This study was not designed to compare the outcomes in these two treatment cohorts. Based on initial data from CheckMate-142, the FDA in July 2017 granted accelerated approval to OPDIVO® for the treatment of patients with MisMatch Repair deficient (dMMR) and MicroSatellite Instability-High (MSI-H) metastatic CRC, that has progressed, following treatment with a Fluoropyrimidine, Oxaliplatin, and Irinotecan.
This review provides an update on outcomes with monotherapy and immunotherapy combination. In the OPDIVO® monotherapy group which included 74 patients, the updated analysis at 21 months showed a response rate was 34%, with 9% being complete responses, and the disease control rate was 62%. The median duration of response has not been reached in the overall cohort of patients and among those responding, 64% had responses lasting at least 1 year. Longer follow up resulted in deepening response rates. The median Progression Free Survival (PFS) for the entire cohort was 6.6 months. Clinical Benefit was seen regardless of PD-L1 expression, BRAF mutation status, KRAS mutation status, and clinical history of Lynch Syndrome.
In the combination immunotherapy group, the median follow up was 13.4 months and the authors of this analysis, André, et al., compared the results of this cohort with those of the OPDIVO® monotherapy group, for the same 13.4 month median follow up period. The most common prior therapies included Fluoropyrimidine (99%), Oxaliplatin (93%) and Irinotecan (73%). Of the 119 patients who received this combination immunotherapy, 76% had 2 or more prior lines of therapy. The Objective Response Rate with a combination of OPDIVO® and YERVOY® was 55%, with 3.4% Complete Responses, and the Disease Control Rate was 80%. About 78% of the patients had reduction in tumor burden with combination immunotherapy. The median time to response was 2.8 months and the median Duration of Response has not yet been reached. Among patients who responded to the combination, 94% had ongoing responses at the time of data cutoff and 63% of the cohort receiving combination immunotherapy remained on treatment. These responses were noted regardless of PD-L1 expression, BRAF or KRAS mutation status, or clinical history of Lynch syndrome. The PFS and Overall Survival with combination immune checkpoint inhibitor therapy at 12 months were 71% and 85%, respectively. There were statistically significant and clinically meaningful improvements in quality-of-life measurements as well.
These data from the CheckMate-142 study support the use of OPDIVO® as a single agent or in combination with YERVOY®, for the treatment of patients with previously treated DNA MisMatch Repair-Deficient/MicroSatellite Instability-High (MSI-H) metastatic CRC.
1. Overman MJ, Bergamo F, McDermott RS, et al. Nivolumab in patients with DNA mismatch repair-deficient/microsatellite instability-high (dMMR/MSI-H) metastatic colorectal cancer (mCRC): Long-term survival according to prior line of treatment from CheckMate-142. Overman MJ, Bergamo F, McDermott RS, et al. J Clin Oncol 36, 2018 (suppl 4S; abstr 554)
2. Nivolumab + ipilimumab combination in patients with DNA mismatch repair-deficient/microsatellite instability-high (dMMR/MSI-H) metastatic colorectal cancer (mCRC): First report of the full cohort from CheckMate-142. André T, Lonardi S, Wong M, et al. J Clin Oncol 36, 2018 (suppl 4S; abstr 553)