SUMMARY:The U. S. Food and Drug Administration on February 3, 2015 granted accelerated approval to IBRANCE® (Palbociclib), for use in combination with FEMARA® (Letrozole), for the treatment of postmenopausal women with Estrogen Receptor (ER)-positive, Human Epidermal growth factor Receptor 2 (HER2) negative advanced Breast Cancer, as initial endocrine-based therapy for their metastatic disease. Approximately 60% of the breast tumors express Estrogen Receptors and these patients are often treated with anti-estrogen therapy as first line treatment.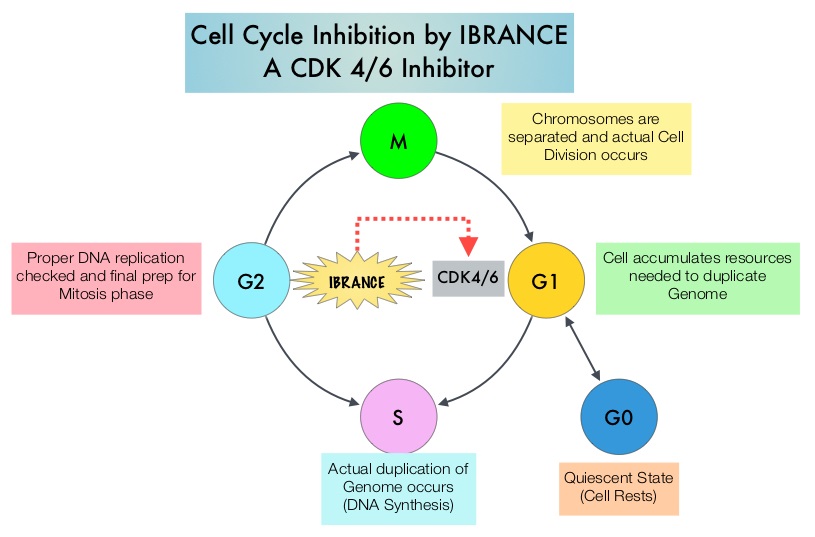 Cyclin Dependent Kinases (CDK) play a very important role to facilitate orderly and controlled progression of the cell cycle. Genetic alterations in these kinases and their regulatory proteins have been implicated in various malignancies. IBRANCE® is a reversible, oral, small molecule inhibitor of Cyclin Dependent Kinases (CDK) 4/6 and is the first CDK inhibitor approved by the FDA. This agent in pre-clinical studies was noted to reduce cellular proliferation of Estrogen Receptor positive Breast Cancer cell lines by blocking progression of cells from G1 into S phase of the cell cycle and was also noted to be synergistic with anti-estrogens. The approval of IBRANCE® was based on an open-label, randomized, multicenter, phase II study which included postmenopausal women with ER-positive, HER2-negative, advanced (locally advanced or metastatic) Breast Cancer who had not received previous systemic treatment for advanced disease. This trial enrolled and randomly assigned 165 patients, to receive either IBRANCE® 125 mg PO daily for 21 consecutive days, followed by 7 days off treatment) plus FEMARA® (2.5 mg PO daily continuously throughout the 28-day cycle (N=84) or FEMARA® alone (N=81). Among the 165 patients, 43% had received chemotherapy and 33% had received anti-hormonal therapy as a neoadjuvant or adjuvant treatment. Fortynine percent (49%) of patients had no prior systemic therapy in the neoadjuvant or adjuvant setting. The majority of patients (98%) had stage IV disease, 48% had visceral disease and 17% had bone only disease. The primary endpoint was investigator-assessed Progression Free Survival. The median PFS was 20.2 months in the IBRANCE® plus FEMARA® group and 10.2 months in the FEMARA® alone group (HR=0.488, P=0•0004). In patients with measurable disease, the Overall Response rate was higher in the IBRANCE® plus FEMARA® group compared to the FEMARA® alone group (55% versus 39%, P=0.04). The median duration of response for those who had a partial or complete response was 20.3 months with a combination of IBRANCE® plus FEMARA® vs 11.1 months with FEMARA® alone. The most common Grade 3–4 adverse events in the IBRANCE® plus FEMARA® group were neutropenia and leucopenia but without any cases of neutropenic fever. Unlike chemotherapy, the duration of neutropenia was brief with rapid recovery of blood counts. The authors concluded that the addition of IBRANCE® to FEMARA® significantly improved Progression Free Survival in women with advanced Estrogen Receptor positive and HER2-negative breast cancer. This combination may therefore be a reasonable treatment option in this patient group, soon after metastatic disease is diagnosed. Biomarkers expression (cyclinD1 amplification and/or loss of p16) had no impact on outcomes suggesting that the biomarker for IBRANCE® may be the Estrogen Receptor itself, rather than CDK4/6 kinases. Finn RS, Crown JP, Lang I, et al. Lancet Oncol 2015; 16:25-35
Cyclin Dependent Kinases (CDK) play a very important role to facilitate orderly and controlled progression of the cell cycle. Genetic alterations in these kinases and their regulatory proteins have been implicated in various malignancies. IBRANCE® is a reversible, oral, small molecule inhibitor of Cyclin Dependent Kinases (CDK) 4/6 and is the first CDK inhibitor approved by the FDA. This agent in pre-clinical studies was noted to reduce cellular proliferation of Estrogen Receptor positive Breast Cancer cell lines by blocking progression of cells from G1 into S phase of the cell cycle and was also noted to be synergistic with anti-estrogens. The approval of IBRANCE® was based on an open-label, randomized, multicenter, phase II study which included postmenopausal women with ER-positive, HER2-negative, advanced (locally advanced or metastatic) Breast Cancer who had not received previous systemic treatment for advanced disease. This trial enrolled and randomly assigned 165 patients, to receive either IBRANCE® 125 mg PO daily for 21 consecutive days, followed by 7 days off treatment) plus FEMARA® (2.5 mg PO daily continuously throughout the 28-day cycle (N=84) or FEMARA® alone (N=81). Among the 165 patients, 43% had received chemotherapy and 33% had received anti-hormonal therapy as a neoadjuvant or adjuvant treatment. Fortynine percent (49%) of patients had no prior systemic therapy in the neoadjuvant or adjuvant setting. The majority of patients (98%) had stage IV disease, 48% had visceral disease and 17% had bone only disease. The primary endpoint was investigator-assessed Progression Free Survival. The median PFS was 20.2 months in the IBRANCE® plus FEMARA® group and 10.2 months in the FEMARA® alone group (HR=0.488, P=0•0004). In patients with measurable disease, the Overall Response rate was higher in the IBRANCE® plus FEMARA® group compared to the FEMARA® alone group (55% versus 39%, P=0.04). The median duration of response for those who had a partial or complete response was 20.3 months with a combination of IBRANCE® plus FEMARA® vs 11.1 months with FEMARA® alone. The most common Grade 3–4 adverse events in the IBRANCE® plus FEMARA® group were neutropenia and leucopenia but without any cases of neutropenic fever. Unlike chemotherapy, the duration of neutropenia was brief with rapid recovery of blood counts. The authors concluded that the addition of IBRANCE® to FEMARA® significantly improved Progression Free Survival in women with advanced Estrogen Receptor positive and HER2-negative breast cancer. This combination may therefore be a reasonable treatment option in this patient group, soon after metastatic disease is diagnosed. Biomarkers expression (cyclinD1 amplification and/or loss of p16) had no impact on outcomes suggesting that the biomarker for IBRANCE® may be the Estrogen Receptor itself, rather than CDK4/6 kinases. Finn RS, Crown JP, Lang I, et al. Lancet Oncol 2015; 16:25-35
Tag: Breast Cancer
Adjuvant Paclitaxel and Trastuzumab for Node-Negative, HER2-Positive Breast Cancer
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their life time. Approximately, 233,000 new cases of invasive breast cancer were diagnosed in 2014 and 40,000 women died of the disease. The HER or erbB family of receptors consist of HER1, HER2, HER3 and HER4. Approximately 15%-20% of invasive breast cancers overexpress HER2/neu oncogene, which is a negative predictor of outcomes without systemic therapy. HERCEPTIN® (Trastuzumab) is a humanized monoclonal antibody targeting HER2.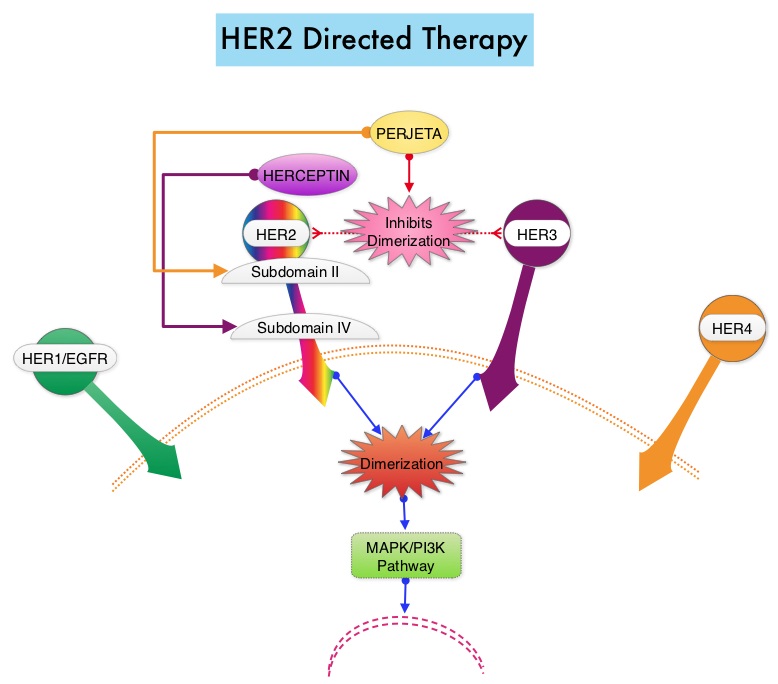 HERCEPTIN® binds to subdomain IV of the HER2 extracellular domain and blocks the downstream cell signaling pathways (PI3K-AKT pathway) and induces Antibody Dependent Cellular Cytotoxicity (ADCC). HERCEPTIN® in combination with chemotherapy has been proven to significantly improve Progression Free Survival and Overall Survival in patients with advanced breast cancer. Adjuvant chemotherapy in combination with HERCEPTIN® has been shown to reduce the relative risk of relapse by 52% and relative risk of death by 33%. The National Comprehensive Cancer Network (NCCN) has recommended adjuvant chemotherapy with HERCEPTIN® for patients with small, HER positive, node-negative tumors, including those with T1bN0 tumors, even though there are little or no data supporting this recommendation, because these patients are generally not included in adjuvant therapy studies. Further, the chemotherapy regimens often recommended (ACTH, TCH) along with HERCEPTIN® are relatively toxic. The authors in this study chose a less toxic chemotherapy regimen than the regimens often recommended for those patients with high risk disease. In this multicenter, investigator initiated study, 406 patients with tumors measuring up to 3 cm in greatest dimension received weekly treatment with TAXOL® (Paclitaxel) and HERCEPTIN (Trastuzumab) for 12 weeks, followed by 9 months of HERCEPTIN® monotherapy. Close to 50% of the patients had tumors 1 cm in diameter or less, about 40% of the patients had tumors 1-2 cm in diameter and majority of the tumors (56%) were high grade. Treatment regimen consisted of TAXOL® 80 mg/m2 IV weekly, for 12 weeks and HERCEPTIN® 4 mg/kg loading dose IV on day 1, followed by 2 mg/kg weekly, for a total of 12 doses followed by HERCEPTIN® 6 mg/kg every 3 weeks for an additional 40 weeks, for a total of 52 weeks of treatment with HERCEPTIN®. Patients who underwent lumpectomy received either partial breast radiation before the initiation of the therapy, or radiation of the whole breast, following completion of treatment with TAXOL®. Treatment with HERCEPTIN® was continued during the time patient was receiving radiation therapy. Adjuvant hormonal therapy was recommended for women with hormone-receptor positive tumors after the completion of TAXOL® treatment. The primary end point was survival free from invasive disease. The median follow up period was 4 years. The 3-year rate of survival free from invasive disease was 98.7%. Treatment was very well tolerated with a low incidence of heart failure (0.5%) and neuropathy. The authors concluded that a less toxic regimen such as HERCEPTIN® given along with weekly TAXOL® has significant efficacy, decreasing the risk of recurrence in this patient group, most notable during the first three years after diagnosis. They also point out that the risk of recurrence of breast cancer is greatest during the first 3-5 years after diagnosis and it would seem unlikely that a different chemotherapy regimen administered with HERCEPTIN® would impact the risk of late recurrences. Tolaney SM, Barry WT, Dang CT, et al. N Engl J Med 2015;372:134-141
HERCEPTIN® binds to subdomain IV of the HER2 extracellular domain and blocks the downstream cell signaling pathways (PI3K-AKT pathway) and induces Antibody Dependent Cellular Cytotoxicity (ADCC). HERCEPTIN® in combination with chemotherapy has been proven to significantly improve Progression Free Survival and Overall Survival in patients with advanced breast cancer. Adjuvant chemotherapy in combination with HERCEPTIN® has been shown to reduce the relative risk of relapse by 52% and relative risk of death by 33%. The National Comprehensive Cancer Network (NCCN) has recommended adjuvant chemotherapy with HERCEPTIN® for patients with small, HER positive, node-negative tumors, including those with T1bN0 tumors, even though there are little or no data supporting this recommendation, because these patients are generally not included in adjuvant therapy studies. Further, the chemotherapy regimens often recommended (ACTH, TCH) along with HERCEPTIN® are relatively toxic. The authors in this study chose a less toxic chemotherapy regimen than the regimens often recommended for those patients with high risk disease. In this multicenter, investigator initiated study, 406 patients with tumors measuring up to 3 cm in greatest dimension received weekly treatment with TAXOL® (Paclitaxel) and HERCEPTIN (Trastuzumab) for 12 weeks, followed by 9 months of HERCEPTIN® monotherapy. Close to 50% of the patients had tumors 1 cm in diameter or less, about 40% of the patients had tumors 1-2 cm in diameter and majority of the tumors (56%) were high grade. Treatment regimen consisted of TAXOL® 80 mg/m2 IV weekly, for 12 weeks and HERCEPTIN® 4 mg/kg loading dose IV on day 1, followed by 2 mg/kg weekly, for a total of 12 doses followed by HERCEPTIN® 6 mg/kg every 3 weeks for an additional 40 weeks, for a total of 52 weeks of treatment with HERCEPTIN®. Patients who underwent lumpectomy received either partial breast radiation before the initiation of the therapy, or radiation of the whole breast, following completion of treatment with TAXOL®. Treatment with HERCEPTIN® was continued during the time patient was receiving radiation therapy. Adjuvant hormonal therapy was recommended for women with hormone-receptor positive tumors after the completion of TAXOL® treatment. The primary end point was survival free from invasive disease. The median follow up period was 4 years. The 3-year rate of survival free from invasive disease was 98.7%. Treatment was very well tolerated with a low incidence of heart failure (0.5%) and neuropathy. The authors concluded that a less toxic regimen such as HERCEPTIN® given along with weekly TAXOL® has significant efficacy, decreasing the risk of recurrence in this patient group, most notable during the first three years after diagnosis. They also point out that the risk of recurrence of breast cancer is greatest during the first 3-5 years after diagnosis and it would seem unlikely that a different chemotherapy regimen administered with HERCEPTIN® would impact the risk of late recurrences. Tolaney SM, Barry WT, Dang CT, et al. N Engl J Med 2015;372:134-141
Sentinel Lymph Node Biopsy for Patients with Early-Stage Breast Cancer American Society of Clinical Oncology Clinical Practice Guideline Update
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately, 233,000 new cases of invasive breast cancer will be diagnosed in 2014 and 40,000 women will die of the disease. Surgical resection of the axillary lymph nodes in addition to potentially removing cancer that may have spread, also facilitates staging of breast cancer.  The sentinel node is the first lymph node(s) to which cancer cells are most likely to metastasize from a primary tumor. With the introduction of intraoperative lymphatic mapping in the 1990s, Sentinel Lymph Node Biopsy (SLNB) has gained general acceptance and is the preferred procedure in appropriate circumstances. Unlike Axillary Lymph Node Dissection (ALND), SLNB is associated with a lower incidence of Lymphedema, seroma at the surgery site, paresthesias and restriction of joint movement. Nine randomized clinical trials have not shown any difference in mortality among patients who underwent ALND or SLNB for either lymph node metastases or negative sentinel lymph nodes, validating Sentinel Lymph Node Biopsy (SLNB). The American Society of Clinical Oncology (ASCO) first published guidelines on the use of SLNB for patients with early stage breast cancer in 2005, based on one randomized clinical trial. Since then, additional information from 9 randomized clinical trials and13 cohort studies pertinent to SLNB and ALND has resulted in this ASCO Clinical Practice Guideline Update.
The sentinel node is the first lymph node(s) to which cancer cells are most likely to metastasize from a primary tumor. With the introduction of intraoperative lymphatic mapping in the 1990s, Sentinel Lymph Node Biopsy (SLNB) has gained general acceptance and is the preferred procedure in appropriate circumstances. Unlike Axillary Lymph Node Dissection (ALND), SLNB is associated with a lower incidence of Lymphedema, seroma at the surgery site, paresthesias and restriction of joint movement. Nine randomized clinical trials have not shown any difference in mortality among patients who underwent ALND or SLNB for either lymph node metastases or negative sentinel lymph nodes, validating Sentinel Lymph Node Biopsy (SLNB). The American Society of Clinical Oncology (ASCO) first published guidelines on the use of SLNB for patients with early stage breast cancer in 2005, based on one randomized clinical trial. Since then, additional information from 9 randomized clinical trials and13 cohort studies pertinent to SLNB and ALND has resulted in this ASCO Clinical Practice Guideline Update.
The following recommendations were made by the American Society of Clinical Oncology panel of experts:
1) Women without sentinel lymph node (SLN) metastases should not undergo Axillary Lymph Node Dissection (ALND).
2) Women with one to two metastatic SLNs planning to undergo breast conserving surgery with whole breast radiotherapy should not undergo ALND (in most cases).
3) Women with SLN metastases who will undergo mastectomy should be offered ALND.
4) Women with operable breast cancer and multicentric tumors, those with ductal carcinoma in situ (DCIS) who will undergo mastectomy, those who previously underwent breast and/or axillary surgery and those who received preoperative/neoadjuvant systemic therapy, may be offered SLNB.
5) Women who have large or locally advanced invasive breast cancer (tumor size T3/T4), inflammatory breast cancer, or DCIS (when breast-conserving surgery is planned) or are pregnant, should not undergo SLNB.
Lyman GH, Temin S, Edge SB, et al. J Clin Oncol 2014;32:1365-1383
Final overall survival analysis from the Cleopatra study of first-line pertuzumab, trastuzumab and docetaxel in patients with HER2-positive metastatic breast cancer
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately, 233,000 new cases of invasive breast cancer will be diagnosed in 2014 and 40,000 women will die of the disease. The HER or erbB family of receptors consist of HER1, HER2, HER3 and HER4. Approximately 15%-20% of invasive breast cancers overexpress HER2/neu oncogene, which is a negative predictor of outcomes without systemic therapy. HERCEPTIN® (Trastuzumab) is a humanized monoclonal antibody targeting HER2. Trastuzumab binds to subdomain IV of the HER2 extracellular domain and blocks the downstream cell signaling pathways (PI3K-AKT pathway) and induces Antibody Dependent Cellular Cytotoxicity (ADCC). HERCEPTIN® in combination with chemotherapy has been proven to significantly improve Progression Free Survival and Overall Survival in patients with advanced breast cancer. Despite this benefit, majority of these patients develop progressive disease within 18 months. The tumors in these patients continue to express HER2 although the lower sensitivity to HER2 targeted agents has been attributed to HER2 independent escape mechanisms. Treatment strategies for this patient population have included switching chemotherapy in subsequent lines of treatment and continuing HERCEPTIN®, combining another HER2 targeted agent, Lapatinib (TYKERB®) with Capecitabine (XELODA®) and dual HER2 inhibition with a combination of HERCEPTIN® and TYKERB®.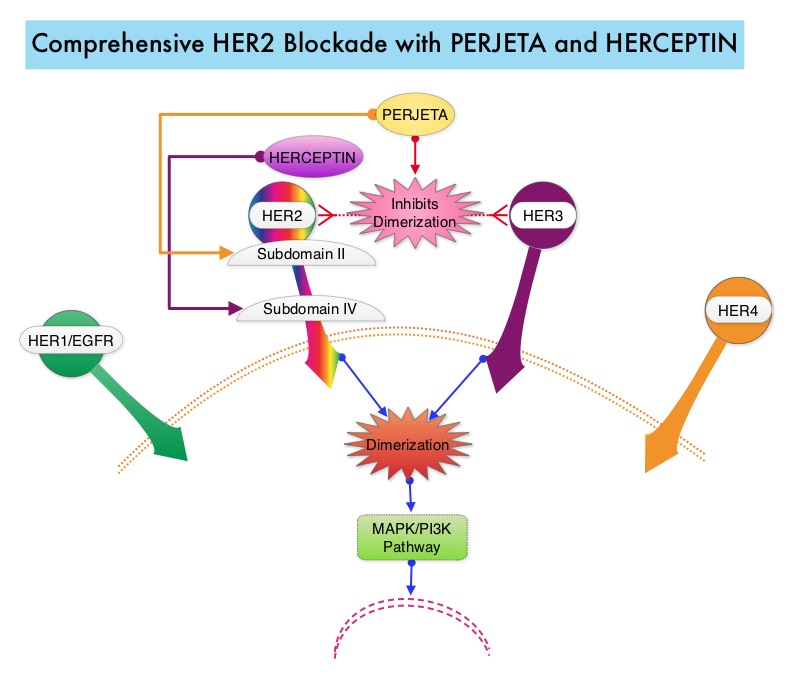 PERJETA® (Pertuzumab) is a recombinant humanized monoclonal antibody that binds to the HER2 at a different epitope of the HER2 extracellular domain (subdomain II) compared to HERCEPTIN® and prevents the dimerization of HER2 with HER3 receptor. PERJETA® stimulates antibody-dependent, cell-mediated cytotoxicity similar to HERCEPTIN®. By combining HERCEPTIN® and PERJETA®, a more comprehensive blockade of HER2 signaling can be accomplished, as these two agents bind to different HER2 epitopes and may complement each other and improve efficacy, as was demonstrated in early phase trials. The CLEOPATRA trial is a phase III study in which 808 treatment naive HER positive metastatic breast cancer patients, were randomly assigned to receive either HERCEPTIN® plus Docetaxel or this two drug combination given along with PERJETA®. PERJETA® was given as an 840 mg loading dose followed by a 420 mg maintenance dose, HERCEPTIN® was given as an 8 mg/kg loading dose followed by a 6 mg/kg maintenance dose and Docetaxel was given at 75 mg/m2 for at least 6 cycles. Treatment was administered every 3 weeks and continued until disease progression. The primary end point of this study was Progression Free Survival and secondary end points included Overall Survival, objective response rate and safety. A previous analysis performed in May 2012 showed that the addition of PERJETA® to the combination of HERCEPTIN® and Docetaxel significantly prolonged Progression Free Survival compared to HERCEPTIN® plus Docetaxel alone (18.5 months vs 12.4 months) but the median overall survival had not been reached then. In this final Overall Survival analysis, at a median follow up of 50 months, median Overall Survival was 56.5 months with the PERJETA® combination compared to 40.8 months in the non-PERJETA® group (hazard ratio [HR] = 0.68; P=0.0002). This meant that adding PERJETA® to HERCEPTIN® and Docetaxel increased the median Overall Survival by 15.7 months. The increase in Progression Free Survival by 6.3 months with the PERJETA® combination, was again maintained (HR = 0.68, P < 0.0001) at the time of the final analysis. The incidence of symptomatic left ventricular dysfunction as well as declines in left ventricular ejection fraction, were rare and similar between the two treatment groups. Based on the CLEOPATRA study, women with HER positive metastatic breast cancer, should be considered candidates, for treatment with a combination of PERJETA®, HERCEPTIN® and Docetaxel. Swain S, Kim S, Cortes J, et al. Presented at: the 2014 Congress of the European Society of Medical Oncology; September 26-30, 2014; Madrid, Spain. Abstract 350O
PERJETA® (Pertuzumab) is a recombinant humanized monoclonal antibody that binds to the HER2 at a different epitope of the HER2 extracellular domain (subdomain II) compared to HERCEPTIN® and prevents the dimerization of HER2 with HER3 receptor. PERJETA® stimulates antibody-dependent, cell-mediated cytotoxicity similar to HERCEPTIN®. By combining HERCEPTIN® and PERJETA®, a more comprehensive blockade of HER2 signaling can be accomplished, as these two agents bind to different HER2 epitopes and may complement each other and improve efficacy, as was demonstrated in early phase trials. The CLEOPATRA trial is a phase III study in which 808 treatment naive HER positive metastatic breast cancer patients, were randomly assigned to receive either HERCEPTIN® plus Docetaxel or this two drug combination given along with PERJETA®. PERJETA® was given as an 840 mg loading dose followed by a 420 mg maintenance dose, HERCEPTIN® was given as an 8 mg/kg loading dose followed by a 6 mg/kg maintenance dose and Docetaxel was given at 75 mg/m2 for at least 6 cycles. Treatment was administered every 3 weeks and continued until disease progression. The primary end point of this study was Progression Free Survival and secondary end points included Overall Survival, objective response rate and safety. A previous analysis performed in May 2012 showed that the addition of PERJETA® to the combination of HERCEPTIN® and Docetaxel significantly prolonged Progression Free Survival compared to HERCEPTIN® plus Docetaxel alone (18.5 months vs 12.4 months) but the median overall survival had not been reached then. In this final Overall Survival analysis, at a median follow up of 50 months, median Overall Survival was 56.5 months with the PERJETA® combination compared to 40.8 months in the non-PERJETA® group (hazard ratio [HR] = 0.68; P=0.0002). This meant that adding PERJETA® to HERCEPTIN® and Docetaxel increased the median Overall Survival by 15.7 months. The increase in Progression Free Survival by 6.3 months with the PERJETA® combination, was again maintained (HR = 0.68, P < 0.0001) at the time of the final analysis. The incidence of symptomatic left ventricular dysfunction as well as declines in left ventricular ejection fraction, were rare and similar between the two treatment groups. Based on the CLEOPATRA study, women with HER positive metastatic breast cancer, should be considered candidates, for treatment with a combination of PERJETA®, HERCEPTIN® and Docetaxel. Swain S, Kim S, Cortes J, et al. Presented at: the 2014 Congress of the European Society of Medical Oncology; September 26-30, 2014; Madrid, Spain. Abstract 350O
Circulating Tumor Cells and Response to Chemotherapy in Metastatic Breast Cancer SWOG S0500
SUMMARY: Circulating tumor cells (CTCs) are epithelial cells that are shed into the circulation from a primary or metastatic tumor. After being shed, CTCs can remain in the circulation or undergo apoptosis. Evaluation of CTCs during the course of disease and treatment has prognostic value. Because of the very low concentrations of CTCs (1 CTC in the background of millions of normal hematopoietic cells) in the peripheral blood, different technologies have been developed that will allow enrichment and detection of these CTCs.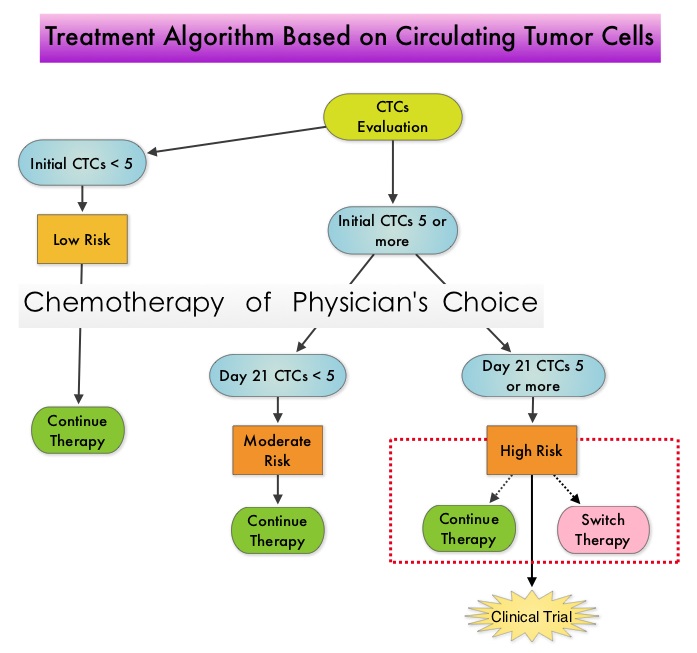 One such technology is the CellSearch® system which is the first FDA-approved test for CTC assessment, in the peripheral blood of Metastatic Breast Cancer (MBC) patients. This automated system is able to enrich the peripheral blood sample with CTCs and the cells then are fluorescently stained for CytoKeratins (CK8,18 and 19), Common Leukocyte Antigen (CD45) and a nuclear dye (DAPI). CTCs are identified when they are CK and DAPI positive and CD45 negative. In essence, CTC assessment, is a real time, peripheral blood evaluation (“Liquid Biopsy”) in MBC patients. Previously published studies have concluded that in patients with MBC, increased levels of CTCs prior to administration of a new therapy was associated with poor outcomes and failure of CTCs to drop to below 5 CTCs per 7.5 mL of peripheral blood at 3- 5 weeks after systemic therapy initiation, predicted worse Progression Free Survival (PFS) and Overall Survival (OS), compared to those who did not have increased CTCs at baseline or had increased CTCs at baseline and but not at 3-5 weeks after therapy. With this background, a randomized study was conducted to assess whether changing treatment after one cycle of first line chemotherapy, in those with a persistent increase in CTCs, improved OS. Evaluable patients were initially divided into two groups – Group A (N=276) included patients who did not have increased CTCs at baseline and Group B (N=288) included patients who had 5 or more CTCs per 7.5 mL of peripheral blood. Eligible patients were chemotherapy naïve for MBC and were treated with single agent chemotherapy. The choice of chemotherapy was at the discretion of the attending physician. Patients in Group A remained on initial therapy until disease progression whereas patients in Group B had CTC evaluation at Day 22 and those with decreased CTCs remained on the initial therapy (N=165). Patients who had persistently increased CTCs at Day 22 (N=123), were then randomly assigned to either continue the initial therapy (Group C1) or switch to a different chemotherapy regimen (Group C2). The median Overall Survival for Groups A, B, and C (C1 and C2 combined) were 35 months, 23 months, and 13 months, respectively (P <0.001). There was no difference in median Overall Survival between Groups C1 and C2 (10.7 and 12.5 months, respectively (P = 0.98). The authors concluded that CTCs in patients with Metastatic Breast Cancer receiving first line chemotherapy has significant prognostic value and changing to a different chemotherapy regimen based on persistently increased CTCs after 3 weeks of first line chemotherapy, had no impact in prolonging Overall Survival. This group of patients (C1 and C2) should be encouraged to enroll in clinical trials as standard chemotherapy may not be as effective. CTC count can prognosticate Progression Free Survival and Overall Survival early in the treatment course thereby allowing customized care. Further, CTC enumeration, unlike mucin based serum biomarkers such as CEA and CA15-3, better correlates with clinical and pathological characteristics of the disease. Smerage JB, Barlow WE, Hortobagyi GN, et al. DOI: 10.1200/JCO.2014.56.2561
One such technology is the CellSearch® system which is the first FDA-approved test for CTC assessment, in the peripheral blood of Metastatic Breast Cancer (MBC) patients. This automated system is able to enrich the peripheral blood sample with CTCs and the cells then are fluorescently stained for CytoKeratins (CK8,18 and 19), Common Leukocyte Antigen (CD45) and a nuclear dye (DAPI). CTCs are identified when they are CK and DAPI positive and CD45 negative. In essence, CTC assessment, is a real time, peripheral blood evaluation (“Liquid Biopsy”) in MBC patients. Previously published studies have concluded that in patients with MBC, increased levels of CTCs prior to administration of a new therapy was associated with poor outcomes and failure of CTCs to drop to below 5 CTCs per 7.5 mL of peripheral blood at 3- 5 weeks after systemic therapy initiation, predicted worse Progression Free Survival (PFS) and Overall Survival (OS), compared to those who did not have increased CTCs at baseline or had increased CTCs at baseline and but not at 3-5 weeks after therapy. With this background, a randomized study was conducted to assess whether changing treatment after one cycle of first line chemotherapy, in those with a persistent increase in CTCs, improved OS. Evaluable patients were initially divided into two groups – Group A (N=276) included patients who did not have increased CTCs at baseline and Group B (N=288) included patients who had 5 or more CTCs per 7.5 mL of peripheral blood. Eligible patients were chemotherapy naïve for MBC and were treated with single agent chemotherapy. The choice of chemotherapy was at the discretion of the attending physician. Patients in Group A remained on initial therapy until disease progression whereas patients in Group B had CTC evaluation at Day 22 and those with decreased CTCs remained on the initial therapy (N=165). Patients who had persistently increased CTCs at Day 22 (N=123), were then randomly assigned to either continue the initial therapy (Group C1) or switch to a different chemotherapy regimen (Group C2). The median Overall Survival for Groups A, B, and C (C1 and C2 combined) were 35 months, 23 months, and 13 months, respectively (P <0.001). There was no difference in median Overall Survival between Groups C1 and C2 (10.7 and 12.5 months, respectively (P = 0.98). The authors concluded that CTCs in patients with Metastatic Breast Cancer receiving first line chemotherapy has significant prognostic value and changing to a different chemotherapy regimen based on persistently increased CTCs after 3 weeks of first line chemotherapy, had no impact in prolonging Overall Survival. This group of patients (C1 and C2) should be encouraged to enroll in clinical trials as standard chemotherapy may not be as effective. CTC count can prognosticate Progression Free Survival and Overall Survival early in the treatment course thereby allowing customized care. Further, CTC enumeration, unlike mucin based serum biomarkers such as CEA and CA15-3, better correlates with clinical and pathological characteristics of the disease. Smerage JB, Barlow WE, Hortobagyi GN, et al. DOI: 10.1200/JCO.2014.56.2561
Clinical Impact of Delaying Initiation of Adjuvant Chemotherapy in Patients with Breast Cancer
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately, 233,000 new cases of invasive breast cancer will be diagnosed in 2014 and 40,000 women will die of the disease. The HER or erbB family of receptors consist of HER1, HER2, HER3 and HER4. Approximately 15%-20% of invasive breast cancers overexpress HER2/neu oncogene, which is a negative predictor of outcomes, without systemic therapy. Patients with early stage breast cancer often receive adjuvant chemotherapy and this is even more so true for HER positive and triple negative (ER, PR and HER negative) breast cancer patients, who are at an increased risk to develop recurrent disease. Even though majority of the patients start their adjuvant chemotherapy within 4-6 weeks following surgery, the impact of delay in the initiation of adjuvant therapy, on outcomes, has remained unclear. Preclinical models have suggested that there is phase of increased angiogenesis and accelerated growth of micrometastases, as well as development of drug resistant clones, following removal of the primary tumor. Previously published data from a large meta-analysis had suggested that a four week delay in the initiation of adjuvant chemotherapy resulted in a 6% increase in the risk of death and an 8% increase in the risk of relapse. Based on this background information, the authors in this study evaluated the impact of time to initiation of adjuvant chemotherapy, on survival, in patients with various stages and subtypes of early stage breast cancer. In this single institution study, 6,827 women diagnosed with stages I to III breast cancer between 1997 and 2011, were categorized into one of three groups – 30 days or less, 31 to 60 days and 61 days or more, according to the time from definitive surgery to adjuvant chemotherapy. Survival outcomes were then estimated in these three groups. The median follow up was 59.3 months and majority of the patients (84.5%) had stage I or II breast cancer and 15.5% of the patients had stage III disease. The authors noted that outcomes were inferior among patients with stage II and stage III disease when chemotherapy was initiated 61 days or more after surgery, with a 76% increase in the risk of death among patients with stage III disease. This disadvantage was however not noted in patients with stage I disease. Survival estimates based on the tumor sub types revealed that patients with triple negative breast cancer tumors and those with HER-2 positive (Human Epidermal growth factor Receptor- 2) tumors, treated 61 days or more after surgery with HERCEPTIN® (Trastuzumab) based chemotherapy, had the worse survival, compared with those who initiated adjuvant treatment within the first 30 days after surgery. Patients with hormone receptor positive tumors were however not impacted. The authors concluded that delaying the initiation of adjuvant chemotherapy in patients with high risk disease such as those with Stages II and III breast cancer and those with triple negative breast cancer and HER-2 positive tumors, can negatively impact survival outcomes. de Melo Gagliato D, Gonzalez-Angulo AM, Lei X, et al. J Clin Oncol 2014;32:735-744
Phase III trial (Prevention of Early Menopause Study [POEMS]-SWOG S0230) of LHRH analog during chemotherapy (CT) to reduce ovarian failure in early-stage, hormone receptor-negative breast cancer An international Intergroup trial of SWOG, IBCSG, ECOG, and CALGB (Alliance)
SUMMARY:Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately, 233,000 new cases of invasive breast cancer will be diagnosed in 2014 and 40,000 women will die of the disease. Approximately 75% of patients with breast cancer are hormone receptor positive (Estrogen Receptor/Progesterone Receptor positive) and this is a predictor of response to endocrine therapy. In premenopausal woman, the ovary is the main source of estrogen production, whereas in postmenopausal women, the primary source of estrogen is the Aromatase enzyme mediated conversion of androstenedione and testosterone to estrone and estradiol in extragonadal/peripheral tissues. Premature Ovarian Failure (POF) is a common unintended consequence of chemotherapy in premenopausal women. Besides of loss of fertility, which can influence treatment decisions in young women, ovarian failure can lead to menopausal symptoms, sexual dysfunction and loss of bone density. 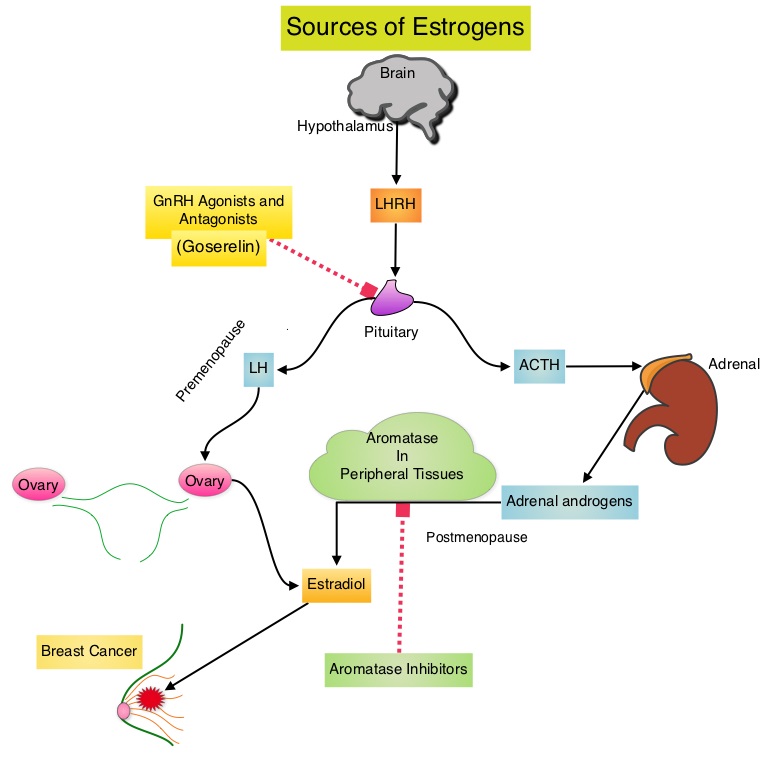 POEMS (Prevention of Early Menopause Study) is a randomized phase III trial designed to evaluate whether the addition of LHRH (Luteinizing Hormone-Releasing Hormone) analog Goserelin (ZOLADEX®), which suppresses the production of estrogens, to Cyclophosphamide based chemotherapy, would reduce POF in breast cancer patients, when compared to chemotherapy alone. Premenopausal patients less than 50 years of age, with hormone negative (ER/PR negative ), Stage I-IIIA breast cancer, scheduled to receive chemotherapy, were randomly assigned to receive standard Cyclophosphamide based chemotherapy with or without monthly ZOLADEX® . Patients in the ZOLADEX® group received 3.6 mg SQ starting 1 week prior to the first dose of chemotherapy. The primary endpoint was ovarian failure at two years (defined as amenorrhea for the prior 6 months AND post-menopausal FSH level). Other endpoints included pregnancy and survival rates. Of the 218 evaluable patients, 135 premenopausal women were evaluable for the primary end point. POF rates were 22% in the chemotherapy alone group and 8% in the ZOLADEX® group (P=0.03). When the definition of POF was more liberal to include EITHER amenorrhea or elevated FSH but not both, POF rates were 45% in the chemotherapy alone group and 20% in the ZOLADEX® group (P=0.006). Among the 218 evaluable patients, more women in the ZOLADEX® group achieved at least one pregnancy (21%) compared to 11% in the chemotherapy alone group (P=0.03). Secondary outcomes also favored the ZOLADEX® group with a Disease free Survival (DFS) rate of 78% in the chemotherapy alone group compared with 89% in the ZOLADEX® group (P=0.04) and Overall Survival (OS) rate of 82% in the chemotherapy alone group compared with 92% in the ZOLADEX® group (P=0.05). The authors concluded that the addition of ZOLADEX® to chemotherapy improved fertility prospects with a lower incidence of Premature Ovarian Failure and more pregnancies. Further, the improved Disease Free Survival and Overall Survival is an important additional perk and prevention of POF with ZOLADEX® may be a consideration not only in premenopausal patients with hormone receptor positive breast cancer but also in other malignancies such as lymphomas, when treated with similar chemotherapeutic agents. Moore HC, Unger JM, Phillips K, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr LBA505)</s
POEMS (Prevention of Early Menopause Study) is a randomized phase III trial designed to evaluate whether the addition of LHRH (Luteinizing Hormone-Releasing Hormone) analog Goserelin (ZOLADEX®), which suppresses the production of estrogens, to Cyclophosphamide based chemotherapy, would reduce POF in breast cancer patients, when compared to chemotherapy alone. Premenopausal patients less than 50 years of age, with hormone negative (ER/PR negative ), Stage I-IIIA breast cancer, scheduled to receive chemotherapy, were randomly assigned to receive standard Cyclophosphamide based chemotherapy with or without monthly ZOLADEX® . Patients in the ZOLADEX® group received 3.6 mg SQ starting 1 week prior to the first dose of chemotherapy. The primary endpoint was ovarian failure at two years (defined as amenorrhea for the prior 6 months AND post-menopausal FSH level). Other endpoints included pregnancy and survival rates. Of the 218 evaluable patients, 135 premenopausal women were evaluable for the primary end point. POF rates were 22% in the chemotherapy alone group and 8% in the ZOLADEX® group (P=0.03). When the definition of POF was more liberal to include EITHER amenorrhea or elevated FSH but not both, POF rates were 45% in the chemotherapy alone group and 20% in the ZOLADEX® group (P=0.006). Among the 218 evaluable patients, more women in the ZOLADEX® group achieved at least one pregnancy (21%) compared to 11% in the chemotherapy alone group (P=0.03). Secondary outcomes also favored the ZOLADEX® group with a Disease free Survival (DFS) rate of 78% in the chemotherapy alone group compared with 89% in the ZOLADEX® group (P=0.04) and Overall Survival (OS) rate of 82% in the chemotherapy alone group compared with 92% in the ZOLADEX® group (P=0.05). The authors concluded that the addition of ZOLADEX® to chemotherapy improved fertility prospects with a lower incidence of Premature Ovarian Failure and more pregnancies. Further, the improved Disease Free Survival and Overall Survival is an important additional perk and prevention of POF with ZOLADEX® may be a consideration not only in premenopausal patients with hormone receptor positive breast cancer but also in other malignancies such as lymphomas, when treated with similar chemotherapeutic agents. Moore HC, Unger JM, Phillips K, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr LBA505)</s
KADCYLA® beneficial for patients with HER2-positive Advanced Breast Cancer who had previously received HERCEPTIN® and TYKERB®.
KADCYLA® (Ado-Trastuzumab Emtansine, T-DM1) is an antibody-drug conjugate (ADC) comprised of the antibody HERCEPTIN® (Trastuzumab) and a chemotherapy agent Emtansine, linked together. Upon binding to the HER2 receptor, KADCYLA® not only inhibits the HER2 signaling pathways but also delivers Emtansine, a microtubule inhibitor, directly inside the tumor cells and destroys them. In the TH3RESA trial, treatment with KADCYLA® significantly improved Progression Free Survival compared to physicians choice, for those patients who had previously received HERCEPTIN® and TYKERB® (Lapatinib) and this therefore makes KADCYLA® the treatment of choice, for this patient population.
A Less Intense Schedule of ZOMETA® for Patients with Metastatic Breast Cancer
Bisphosphonates inhibit osteoclast-mediated bone resorption and both oral and IV bisphosphonates reduce the risk of developing Skeletal Related Events (SRE’s) and delay the time to SRE’s in patients with Breast Cancer with bone metastases. In a study presented at ASCO 2014 meeting, continuing ZOMETA® (Zoledronic acid) for an additional year at the every 12 week schedule was non-inferior to ZOMETA® given every 4 weeks, among patients who had initially received IV bisphosphonates monthly, for one year or longer. This less frequent dosing of ZOMETA® compared with the standard monthly dosing, may be more convenient for the patients and result in less toxicities without compromising efficacy. More information at www.oncoprescribe.com
Efficacy and safety of continued zoledronic acid every 4 weeks versus every 12 weeks in women with bone metastases from breast cancer Results of the OPTIMIZE-2 trial
SUMMARY: Bone is the most common site of metastatic disease, in patients with Breast Cancer. Bisphosphonates inhibit osteoclast-mediated bone resorption and both oral and IV bisphosphonates reduce the risk of developing Skeletal Related Events (SRE’s) and delay the time to SRE’s in patients with Breast Cancer with bone metastases. Bisphosphonates can also reduce bone pain and may improve Quality of life. Of the four bisphosphonates proven to be effective in patients with Breast Cancer with bone metastases, only intravenous Pamidronate (AREDIA®) and Zoledronic acid (ZOMETA®) have been approved in the USA, whereas intravenous and oral Ibandronate and oral Clodronate have been approved in Europe. Both AREDIA® and ZOMETA® are administered every 3 to 4 weeks during the first year, following diagnoses of bone metastases. However, the optimal treatment schedule following this initial phase of treatment has remained unclear. Further, renal toxicity, long bone fractures and OsteoNecrosis of the Jaw (ONJ) have been identified as potential problems with bisphosphonate use. OPTIMIZE-2 is a prospective, randomized, double-blind, multicenter trial, in which the authors evaluated the outcomes of a less intense schedule of ZOMETA® administered every 12 weeks, following one year of the standard initial phase of treatment with bisphosphonates. This study included 403 women with bone metastases from Breast Cancer, who had received 9 or more doses of either intravenous ZOMETA® or AREDIA®, during the first 10-15 months of therapy. The median age was 59 years and patients were randomized (1:1) to receive either ZOMETA® 4 mg IV every 4 weeks (N=200) or every 12 weeks (N=203), for one year. The primary endpoint was Skeletal Related Event (SRE) rate, defined as the proportion of patients with one or more SRE’s (pathologic fractures, spinal cord compression, need for radiotherapy or surgical stabilization of the bone). The primary analysis was non-inferiority, for the difference in SRE rates between the treatment groups. Secondary endpoints included time to first SRE, Skeletal Morbidity Rate (SMR), bone pain score, change in bone turnover markers, and safety. After a median follow up of 11.9 months, the SRE rate was 22% and 23.2% in the ZOMETA® every 4 weeks group and ZOMETA® every 12 weeks group respectively (P=0.724), suggesting that ZOMETA® given every 12 weeks was non-inferior to the q 4 week regimen. The secondary endpoints were comparable as well. More patients had renal toxicities in the ZOMETA® q 4 week group vs q 12 week group (9.6% vs 7.9%, respectively) and two cases (1.0%) of OsteoNecrosis of the Jaw (ONJ) were reported in the ZOMETA® q 4 week group. The authors concluded that the efficacy of continuing ZOMETA® for an additional year at the q 12 week schedule was non-inferior to ZOMETA® given q 4 weeks, among patients who had initially received IV bisphosphonates monthly, for one year or longer. Further the less frequent dosing of ZOMETA® compared with the standard monthly dosing, may be more convenient for the patients and result in less toxicities. Hortobagyi GN, Lipton A, Chew HK, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr LBA9500)
OPTIMIZE-2 is a prospective, randomized, double-blind, multicenter trial, in which the authors evaluated the outcomes of a less intense schedule of ZOMETA® administered every 12 weeks, following one year of the standard initial phase of treatment with bisphosphonates. This study included 403 women with bone metastases from Breast Cancer, who had received 9 or more doses of either intravenous ZOMETA® or AREDIA®, during the first 10-15 months of therapy. The median age was 59 years and patients were randomized (1:1) to receive either ZOMETA® 4 mg IV every 4 weeks (N=200) or every 12 weeks (N=203), for one year. The primary endpoint was Skeletal Related Event (SRE) rate, defined as the proportion of patients with one or more SRE’s (pathologic fractures, spinal cord compression, need for radiotherapy or surgical stabilization of the bone). The primary analysis was non-inferiority, for the difference in SRE rates between the treatment groups. Secondary endpoints included time to first SRE, Skeletal Morbidity Rate (SMR), bone pain score, change in bone turnover markers, and safety. After a median follow up of 11.9 months, the SRE rate was 22% and 23.2% in the ZOMETA® every 4 weeks group and ZOMETA® every 12 weeks group respectively (P=0.724), suggesting that ZOMETA® given every 12 weeks was non-inferior to the q 4 week regimen. The secondary endpoints were comparable as well. More patients had renal toxicities in the ZOMETA® q 4 week group vs q 12 week group (9.6% vs 7.9%, respectively) and two cases (1.0%) of OsteoNecrosis of the Jaw (ONJ) were reported in the ZOMETA® q 4 week group. The authors concluded that the efficacy of continuing ZOMETA® for an additional year at the q 12 week schedule was non-inferior to ZOMETA® given q 4 weeks, among patients who had initially received IV bisphosphonates monthly, for one year or longer. Further the less frequent dosing of ZOMETA® compared with the standard monthly dosing, may be more convenient for the patients and result in less toxicities. Hortobagyi GN, Lipton A, Chew HK, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr LBA9500)
